
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question

Transcribed Image Text:c. What is the composition of the lower boiling compound in Sample 1?
d. What is the composition of the higher boiling compound in Sample 2?
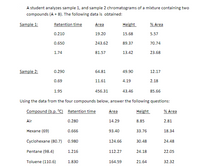
Transcribed Image Text:A student analyzes sample 1, and sample 2 chromatograms of a mixture containing two
compounds (A + B). The following data is obtained:
Sample 1:
Retention time
Area
Height
% Area
0.210
19.20
15.68
5.57
0.650
243.62
89.37
70.74
1.74
81.57
13.42
23.68
Sample 2:
0.290
64.81
49.90
12.17
0.69
11.61
4.19
2.18
1.95
456.31
43.46
85.66
Using the data from the four compounds below, answer the following questions:
Compound (b.p. °C) Retention time
Area
Height
% Area
Air
0.280
14.29
8.85
2.81
Hexane (69)
0.666
93.40
33.76
18.34
Cyclohexane (80.7) 0.980
124.66
30.48
24.48
Pentane (98.4)
1.216
112.27
24.18
22.05
Toluene (110.6)
1.830
164.59
21.64
32.32
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- With the following information for a GC analysis using an internal standard, calculate the percentage of alcohol in the sample. The 1% ethanol standard sample has an ethanol peak area of 711 and a 1-propanol peak area of 9253; 3% is 3536 and 14432 for ethanol and 1-propanol respectively; 5% is 3506 and 10693; 7% is 5874 and 10847 and 9% is 5203 and 7522. The beer sample has an ethanol peak area of 5471 and 1-propanol of 14384. 4.95% 5.15% 5.05% 5.25%arrow_forwardA gas chromatogram of a mixture of toluene and ethyl (a) Measure w1/2 for each peak to the nearest 0.1 mm. When the thickness of the pen trace is significant relative to the length being measured, it is important to take the pen width into account. It is best to measure from the edge of one trace to the corresponding edge of the other trace, as shown at the bottom of the left column. (b) Find the number of theoretical plates and the plate height for each peak.arrow_forwardI have set up my spreadsheet and was able to reproduce the result with a pKa of 4.64. TRUE OR FALSEarrow_forward
- I have set up my spreadsheet using this sample data and reproduced the result with a pKa of 4.64. true or falsearrow_forwardHow to solve the following?arrow_forwardA student developed a new dye called Dye C. It was analyzed against other dyes using paper chromatography. A solution of 1:1 ethyl acetate:hexane was used as the eluent. The resulting chromatogram is shown in the figure. Determine the retention factor (Rf) values of each spot on dye C. (use 2 decimal places) What other dye/s are present in dye C and what is the dye with least number of pigments?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY