
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Show work, thank you!
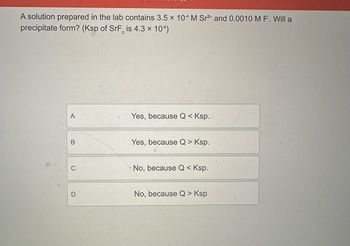
Transcribed Image Text:A solution prepared in the lab contains 3.5 x 104 M Sr2+ and 0.0010 M F. Will a
precipitate form? (Ksp of SrF2 is 4.3 x 10-⁹)
A
B
C
D
Yes, because Q < Ksp.
Yes, because Q > Ksp.
No, because Q < Ksp.
No, because Q > Ksp
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- M HW 2.5 - Blackboard X Mind Tap - Cengage Learning X Bb My Blackboard Content - Black x + 5112480241329813180832311&elSBN=9781305862883&id=1774598910&snapshotid=33... References Submit Answer Use the References to access important values if needed for this question. A student determines the heat of dissolution of solid cesium sulfate using a coffee-cup calorimeter of negligible heat capacity. When 16.2 g of Cs₂SO4(s) is dissolved in 101.00 g of water, the temperature of the solution drops from 25.00 to 23.12 °C. Based on the student's observation, calculate the enthalpy of dissolution of Cs₂SO4(s) in kJ/mol. Assume the specific heat of the solution is 4.184 J/g°C. AH dissolution= kJ/mol ant P Calorimetry - Heat of Solution (Calorimete...: This is group attempt 1 of 10 Autosaved at 9:03 PM [ Q Searcharrow_forwardAutoSave w | homework – Saved to my Mac OFF ... Home Insert Draw Design Layout References Mailings Review View O Tell me R Share O Comments Calibri (Bo. v 11 v A A E vE v E v E E Aa v AaBbCcDdEe AaBbCcD AaBbCcDdE AaBb AaBbCcDdEe Paste BIU V ab x, x A v I v A v No Spacing Normal Heading 1 Heading 2 Title Styles Pane Dictate Sensitivity Please answer the following questions fully and to the best of your ability 1) Please state how you would synthesize the following polymer below and show the mechanism behind the reaction. Note that this also means you need to tell me which molecule you are starting with as well as specifying all conditions required. он OH 2) Will either the polymer or the monomers you used to make it show peaks if IR spectroscopy analysis was done on them, and if so where? How about if UV-vis spectroscopy was performed instead? Page 1 of 1 93 words E English (United States) O Focus 白arrow_forwardme x C Thermo X Cosmet x C Laptops x M Inbox (7 X C Buy TCL X learn.canterbury.ac.uk/ultra/courses/_19016_1/outline/edit/document/_3537106_1?courseld=_19016_1&view=content T K Saturat X ZA a Amazon x C Show Ye X G what's a X Email- 1 X ☆ b The dia x h EX E ct the rato at which + 4. Consider the interconversion of A and B. Suppose in the absence of an enzyme, the forward rate constant KF is 104 s¹ and the reverse rate constant KR is 106 S¹. Calculate the equilibrium constant K. How would the presence of an enzyme affect the value of K? Karrow_forward
- Using the proper conversion factor, convert 8.4 ft? into cm2. ! cm? Save for Later Last saved 1 day ago. Saved work will be auto-submitted on the due date. Auto- submission can take up to 10 minutes. Part 3 The parts of this question must be completed in order. This part will be available Part 4 Te ortc of thic question must he comnleted in order This part will be availablearrow_forwardesc pock Ethanol (C₂H6O) burns in air C₂H60) +O2(g) → CO2(g) + H₂O (1) Balance the equation and determine the volume of air in liters at 35°C and 790 mm Hg required to burn 227 g of ethanol. Assume that air is 21% O₂ by volume. Edit Format Table 12pt Paragraph control ! 1 Q A @ 2 N BIU AT²V W S # 3 X 1 option command E D $ 4 t R C ܀ % 5 F : T V MacBook Pro ➡ A 6 G Y & B 7. H (+) U 0 words *00 8 N J 1 ( 9 K M ) 0 O MOSISO goarrow_forwardWhy should you wear black in the winter and white in the summer? should be an original thought, not Google’s 3-4 sentences asap please!! will rate!! Thank you!!arrow_forward
- Show the work.arrow_forwardThu Ma DSave O OFF a Lab 5_Energy and Matter - Compatibility Mode Draw Design Layout References Mailings View O Tell me Review e Share O Com Arial v 1 v A A Aa v AaBbCcDdt AaBbCcDdEe AaBbCcDdl BIUV ab x, AvevAv E == E v Normal Subtitle No Spacing Styles Pane Dict Heating Curve for Water 120.0 100.0 80.0 60.0 40.0 20.0 0.0 0 2 4 6 8. 10 12 14 16 Time (min) 5. Boiling point of water 99.9 °C 6. Temperature change (AT) 95.4 °C 7. Mass of water 100.0 G Volume of water (1) x 1.00 g/mL 100 mL 8. Joules needed to heat water - + 1479 577 words x English (United States) O Focus MAR étv W Aa 4 DII DD F12 F10 Temperature (°C)arrow_forwardneed percent of water in the hydrate sample for all 3 tries to make sure work is correctarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY