
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
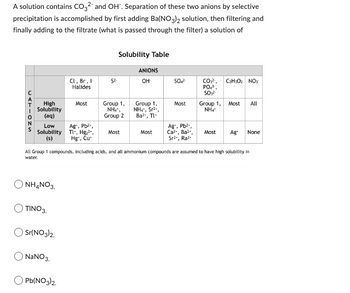
Transcribed Image Text:A solution contains CO32- and OH". Separation of these two anions by selective
precipitation is accomplished by first adding Ba(NO3)2 solution, then filtering and
finally adding to the filtrate (what is passed through the filter) a solution of
CATIONS
High
Solubility
(aq)
Low
Solubility
(s)
NH4NO3.
TINO 3.
Sr(NO3)2.
NaNO3.
Cl, Br, I
Halides
Pb(NO3)2
Most
Ag+, Pb²+,
Tl+, Hg₂2+,
Hg, Cu
52.
Solubility Table
Group 1,
NH₁,
Group 2
Most
ANIONS
OH
Group 1,
NHƯ, Sri
Ba²+, Tl
Most
SO₂²
Most
Ag, Pb²+,
Ca²+, Ba²+,
Sr²+, Raz
CO3²,
PO₂¹
SO3²
All Group 1 compounds, including acids, and all ammonium compounds are assumed to have high solubility in
water.
Group 1,
NH4
C₂H30₂ NO3
Most All
Most Ag+ None
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Some wastewater stream contains 0.015 M Hg22+. Some chemical engineer decides to remove it by precipitating it as Hg2CO3(s). He mixes 100.00 ml of the wasterwater with 100.00 ml of a 2.00 M solution of Na2CO3 and filters the result to remove the solid. How much residual Hg22+ should he expect to find still in the water? (Hint: it should not be 0) Ksp of. Hg2CO3 = 9.0 x 10-15. Also keep in mind that Hg+ is the only ion that exists in solutions as a dimer, i.e. Hg22+, not 2 Hg+ (Hg2CO3→ Hg22+ + CO32-)arrow_forwardA solution contains 8.70x10-³ M potassium hydroxide and 8.16x10-³ M sodium sulfide. Solid iron(III) nitrate is added slowly to this mixture. A. What is the formula of the substance that precipitates first? formula = B. What is the concentration of iron(III) ion when this precipitation first begins? [Fe³+] = ___M PbBr₂ 6.3 x 10-6 Pb(OH)2 2.8 x 10-16arrow_forwardA solution contains 1.13×10-² M nickel(II) acetate and 7.82x10-3 M zinc nitrate. Solid potassium cyanide is added slowly to this mixture. A. What is the formula of the substance that precipitates first? formula = B. What is the concentration of cyanide ion when this precipitation first begins? [CN] = Marrow_forward
- A solution contains 9.92×103 M ammonium carbonate and 9.27x103 M sodium hydroxide. Solid iron(II) acetate is added slowly to this mixture. A. What is the formula of the substance that precipitates first? formula = B. What is the concentration of iron(II) ion when this precipitation first begins? [Fe?*] =[ Marrow_forwardAt a certain temperature, chlorine gas and carbon monoxide react to form carbonyl chloride: Cl2(g) + 2 CO(g) 2 coC(g). When 3.0 x 10-3 M Cl2 is mixed with 0.030 M CO and 9.0 x 10-3 M COCI, the concentration of ccl decreases. Which statement below is true? а. Кс 100 c. Kc = 30 d. Kç > 30 e. Kç = 100 f. Kç < 100 Your answer is incorrect. The correct answer is: Kc < 30arrow_forwardA solution contains 1.45x10-2 M sodium carbonate and 9.94x10-3 M ammonium sulfide. Solid copper(II) nitrate is added slowly to this mixture. A. What is the formula of the substance that precipitates first? formula = B. What is the concentration of copper(II) ion when this precipitation first begins? [Cu²+] = Marrow_forward
- Solid potassium hydroxide is slowly added to 150 mL of a 0.0538 M iron(II) acetate solution. The concentration of hydroxide ion required to just initiate precipitation is M.arrow_forward11c.arrow_forward1. A solution of HCIO, was standardized by dissolving 0.3745 g of primary standard grade HgO in the solution of KBr. The liberated OH required 37.79 mL of the acid to be neutralized. Calculate the molarity of HCIO.. HgOs) + 4Br + H,0 > HgBr. + 20Harrow_forward
- Step 1 Lead(II) ions of Lead(II) nitrate reacts with chloride ion of NaCl to form a PbCl2 precipitate. Pb2+(aq) + 2Cl-(aq) →PbCl2(s) arrow_forward Step 2 The precipitate of PbCl2 starts forming when the reaction quotient (Q=[Pb2+][Cl-]2) is greater than the solubility product of PbCl2. The solubility product of PbCl2 is Ksp=1.17×10-5 To find the concentration of chloride ion that starts the precipitation of PbCl2, equate Q to solubility product and solve for the concentration of Chloride ion. [Pb2+][Cl-]2 =1.17×10-5 [Cl-]2 =1.17×10-5[Pb2+] [Cl-] =1.17×10-5[Pb2+] [Pb2+] = concentration of lead(II) nitrate solution [Cl-] =1.17×10-51.0×10-2=3.4205×10-2 M arrow_forward Step 3 The mass of NaCl needed to start the precipitation of PbCl2 is calculated below. mass of NaCl =[Cl-]×Volume of solution ×Molar mass of NaCl mass of NaCl =3.4205×10-2 M×800 mL ×58.44 gmol mass of NaCl =3.4205×10-2 molL×800 ×10-3 L ×58.44 gmol mass of NaCl =159915.216×10-5 g =1.599 g =1.6arrow_forwardSolid potassium hydroxide is slowly added to 150 mL of a 0.0614 M silver acetate solution. The concentration of hydroxide ion required to just initiate precipitation is ______ M.arrow_forwardA solution is prepared by adding 150.0 mL of a 0.0100 M Mg(NO3)2 solution to 250.0 mL of a 0.100 M NaF solution. Will anything precipitate from this solution and, if so, what is the precipitate? a. No, nothing will precipitate. b. Yes, MgF 2 c. There is not enough information to answer this question. d. Yes, Mg(NO 3 ) 2 e. Yes, NaNO 3arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY