
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
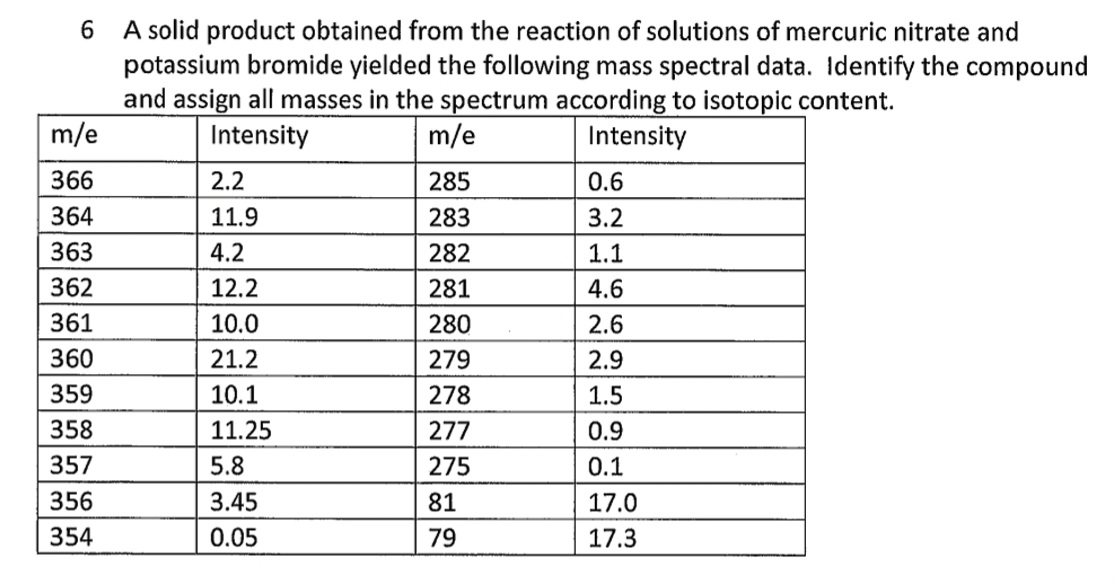
Transcribed Image Text:6
A solid product obtained from the reaction of solutions of mercuric nitrate and
potassium bromide yielded the following mass spectral data. Identify the compound
and assign all masses in the spectrum according to isotopic content.
Intensity
Intensity
m/e
366
364
363
362
361
360
359
358
357
356
354
2.2
11.9
4.2
12.2
10.0
21.2
10.1
11.25
5.8
3.45
0.05
m/e
285
283
282
281
280
279
278
277
275
81
79
0.6
3.2
1.1
4.6
2.6
2.9
1.5
0.9
0.1
17.0
17.3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A solution in part C initially contains (4.06x10^-4) molar SCN¯ and (2.960x10^-3) molar Fe3+. This solution was then measured using the colorimeter, and an absorbance of (4.9800x10^-1) was recorded.The constant determined in part A of the experiment was found to be (3.8200x10^3). Using the data provided above, determine the equilibrium concentration of SCN", [SCN]E in this solution. Note: Your answer is assumed to be reduced to the highest power possible. Your Answer: x10 Answer unitsarrow_forwardA sample was prepared by diluting 1 mL of a sample to 5 mL. The resulting solution has an absorbance of 0.529. Based on the Beer's Law plot prepared for the compound, y = 1.8711x, what is the concentration of the original solution?arrow_forwardWhen the Nickel (II) ion reacts with ammonia, most students notice a colour change occurring. Can you explain what the reaction is? And what colour they would see?arrow_forward
- Ligand X forms a complex with both cobalt and copper, each of which has a maximum absorbance at 510 nm and 645 nm. respectively. A 0.243 g sample containing cobalt and copper was dissolved and diluted to a volume of 100.0 mL. A solution containing ligand X was added to a 50.0 mL aliquot of the sample solution and diluted to a final volume of 100.0 mL. The measured absorbance of the unknown solution was 0.503 at 510 nm and 0.368 at 645 nm, when measured with a 1.00 cm cell. The molar absorptivities of the cobalt and copper complexes at each wavelength are shown in the table. Molar Absorptivity (c. M-'cm-¹) Wavelength [Co² = A, nm [Cu²+] = 510 645 3.125 x10-5 Incorrect Co 2.867 x10-5 3.7630 x 10² What is the concentration of cobalt and copper in the final diluted solution? 1282 Cu 5707 18090 M Marrow_forwardWhen the Nickel (II) ion reacts with ammonia, most students notice a colour change occurring. Can you explain what the reaction is? And what colour they would see?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY