
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
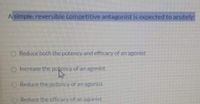
Transcribed Image Text:A simple, reversible competitive antagonist is expected to acutely:
Reduce both the potency and efficacy of an agonist
O Increase the potency of an agonist
O Reduce the potency of an agonist
Reduce the efficacy of an agonist
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 3. If the compound shown is subjected to reaction conditions that will hydrolyze amides one of two products shown will form faster. Which one, A or B? Explain your choice. * for H H3N. HCI -ОН + 'N H2 H20 A Barrow_forwardChoose the set of letters corresponding to the BEST answer. WXY can be produced from cyclopentanamine and CH31 most basic amine most H₂O-soluble least basic amine -NH₂ ZAB CDE [Choose ] [Choose ] [Choose ] [Choose ] FGH -NH₂ IJKarrow_forwardStructure I is a serotonin antagonist. A methyl group has been introduced into analogue II resulting in significantly increased activity. What is a reasonable explanation for the increase in activity observed for structure II. CH చి ి NH FC OMe F;C OMe II.arrow_forward
- What effect does modification of the side chain of the following lead compound have in the modified side chain structure? OH НО. HO None of the answers It makes the agent an agonist O It makes the agent a partial agonist O It makes the agent an antagonist O It makes the agent inactive Lead compound OH Modified side chainarrow_forwardnafina dilute HCI Nat CEN +HCI Predict the products from the reactions of the following amines with sodium nitrile in R-CEN(nrile) + RA a) cyclohexanamine H2 RNH NH VO2 A aniline NHaarrow_forwardWHAT IS THE AMINE SUBSTRATE?arrow_forward
- Which of the following statement(s) MOST closely define an agonist? An agonist is: Group of answer choices A.a substance that brings about a change in biological activity through a chemical reaction B.a specific regulatory molecule in the biological system where a drug interacts C.a drug that binds to a receptor and stimulates cellular activity D.a drug that binds to a receptor and inhibits or opposes cellular activity E. a drug that has strong affinity for the receptor and expresses high efficacyarrow_forwardSelect two substrates and one reagent from each of the given pools to create the amine target molecule.arrow_forwardNeed helparrow_forward
- Classify each amine as a primary, secondary, or tertiary.arrow_forwardIn the design of H2 antagonist, the polar guanidine group was proposed to interact with the antagonist binding region by two hydrogen bonds. Compound A is designed based on modification of compound B and has higher antagonist activity compared to compound B. H2N NH NH2 NH HN Guanidine Guanidine HN What drug design strategy has been used here? O Rigidification O Simplification O Substituent variation O Chain Extensionarrow_forwardSelect the appropriate reagent and TWO possible substrates for the following reductive amination.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY