
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
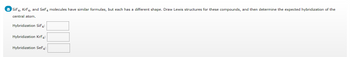
Transcribed Image Text:a SiF4, KrF4, and SeF4 molecules have similar formulas, but each has a different shape. Draw Lewis structures for these compounds, and then determine the expected hybridization of the
central atom.
Hybridization SiF4:
Hybridization KrF4:
Hybridization SeF4:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Fill in the molecular orbitals for the H2 molecule. How do you describe the highest occupied orbital?arrow_forwardhow does molecular orbital theory make it possible to explain both ionic and covalent bonding? The degree of ionic characters in bonding is related to electronegativity in topic 2D. How does electronegativity affect the molecular orbital diagram so that bonds become ionic?arrow_forwardDraw the Lewis structure of CH₃CCH and then choose the appropriate set of hybridization states for the three central atoms. Your answer choice is independent of the orientation of your drawn structure.arrow_forward
- Determine a hybridization and bonding scheme for COIH. Draw its Lewis structure. Determine the hybridization, electron and molecular geometry for the central atom and the number of o and T bonds. Sketch the molecule including overlapping orbitals, label all bonds. (Make sure overlapping orbitals are the appropriate shape orbital that is ½ filled and can share an electron.). Is this molecule polar or non-polar?arrow_forwardIn each of the groups below, all molecules except one share the same orbital hybridization of the central atom. Identify which molecule is different, and name the hybridization for that molecule and the rest of the group. a) NO3", CO32", SO;2", BCI3 b) H2S, SIF4, SeO2, PB13 c) HCN, N2O, NO2", CS2 (Hint: N is the central atom in N2O) d) ClO3", NC13, AlCl3, H3O*arrow_forwardEach of the structures for ClOCl, NCOH, and ClNO has atoms connected in the order given in their formulas. Identify the type of hybridization for the central atom in each compound.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY