
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
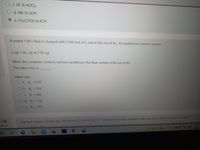
Transcribed Image Text:c. HF & HCIO4
d. HBr & LIOH
e. CH3COOH & KOH
A sealed 1.00L flask is charged with 0.500 mol of I, and 0.500 mol of Br2. An equilibrium reaction ensues:
12 (g) + Br2 (g) =2 IBr (g)
When the container contents achieve equilibrium, the flask contains 0.84 mol of IBr.
The value of K is
Select one:
O a. K= 6.10
O b. K = 2.82
OC K = 882
Od. K= 110
Oe K= 131
en 13
The heat capacity of silver (Ag. 107.9 g maol), is 0.232 J g'K Calculate the energy needed to heat 2e mo
saved
II
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1. Please don't write on a paper. I can't understand handwritten.arrow_forwardConsider the equilibrium system described by the chemical reaction below, which has a value of Kc equal to 1.2×10-4 at a certain temperature. If a solid sample of NH4SH decomposes, what is the equilibrium concentration of NH3 be?arrow_forward4. A. An important reaction in the commercial production of hydrogen is CO(g) + H2O(g) --><-- H2(g) + CO2(g) How will the system at equilibrium shift in each of the five following cases? (Use arrows or write "right" , "left" , or "no shift"). a. Gaseous carbon monoxide is removed b. In rigid reaction container, a catalyst is added. c. The temperature is decreased (the reaction is exothermic). d. The reaction container volume is doubled. e. Hydrogen gas is added. B. The equation above has a Kc value of 7.5 x 102. If 2.0 moles of each reactant and product are put into the same sealed 4.0 L container, determine the concentrations of all species present at equilibrium. Please answer A with sub parts and B.arrow_forward
- Consider the following exothermic reaction: C (s) + 2 Cl2 (g) ⇋⇋ CCl4 (g) How would the equilibrium shift if the reaction mixture is heated? Select one: a. The reaction would shift towards the products. b. The reaction would shift towards the reactants. c. The equilibrium would not be affected.arrow_forwardConsider the following system at equilibrium at 600 K:COCl2(g) =CO(g) + Cl2(g)When some COCl2(g) is added to the equilibrium system at constant temperature: The reaction must: fill in the blank 1 A. Run in the forward direction to restablish equilibrium. B. Run in the reverse direction to restablish equilibrium. C. Remain the same. Already at equilibrium. The concentration of CO will: fill in the blank 2 A. Increase. B. Decrease. C. Remain the same.arrow_forwardConsider the following equilibrium: 2NOC1(g)= 2NO(g) + Cl2(g) with K= 1.6 x 10. 1.00 mole of pure NOC1 and 0.981 mole of pure Cly are placed in a 1.00-L container. Calculate the equilibrium concentration of NO(g). on Select one: O a. 9.81 × 10-M O b. 1.02 M O c.4.04 x 10-3M O d. 2.02 x 10- M O e. 5.71 x 10M nere to search F4 F5 FG FZ F8 F9 F10 F11 F12 PriScr 23 %24 3 6. E R T D G H K C V B N Marrow_forward
- If this reaction is taking place in a 1.0L container, and 1.5 mol of SO2 were added to the reaction, whatwill the new concentrations of all fours gases be when equilibrium is reestablished?arrow_forwardPhosphorus pentachloride decomposes at higher temperatures. PC15 (8) PCl3(g) + Cl₂(g) An equilibrium mixture at some temperature consists of 5.81 g PCI, 208.23 g/mol 4.86 g PC3, 137.33 g/mol 3.59 g Cl₂, 70.91 g/mol in a 1.00-L flask. If you add 1.31 g of Cl₂, how will the equilibrium be affected and what will the concentration of PCI, be when equilibrium is reestablished? O shift left O shift right Ono shift will occur [PCI5] = mol/Larrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY