
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
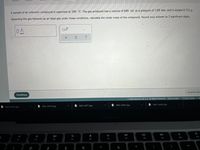
Transcribed Image Text:A sample of an unknown compound is vaporized at 100. °C. The gas produced has a volume of 640. mL at a pressure of 1.00 atm, and it weighs 0.712 g.
Assuming the gas behaves as an ideal gas under these conditions, calculate the molar mass of the compound. Round your answer to 3 significant digits.
x10
mol
Submit Ass
Continue
2022 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center A-
IMG-4471.jpg
IMG-4467.jpg
IMG-4464.jpg
IMG-4474.jpg
IMG-4472.jpg
MacBook Air
DII
DD
80
F11
F7
F8
F9
F10
F4
F5
F6
F1
F2
F3
%
&
%24
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A 9.00 L tank at 2.08 °C is filled with 9.55 g of boron trifluoride gas and 14.3 g of sulfur hexafluoride gas. You can assume both gases behave as ideal gases under these conditions. Calculate the mole fraction and partial pressure of each gas, and the total pressure in the tank. Round each of your answers to 3 significant digits. boron trifluoride sulfur hexafluoride mole fraction: partial pressure: mole fraction: partial pressure: Total pressure in tank: O atm atm atm x10 X 3arrow_forwardA 10.0 L tank at 6.06 °C is filled with 18.1 g of chlorine pentafluoride gas and 5.47 g of boron trifluoride gas. You can assume both gases behave as ideal gases under these conditions. Calculate the mole fraction and partial pressure of each gas, and the total pressure in the tank. Round each of your answers to 3 significant digits. mole fraction: x10 chlorine pentafluoride partial pressure: atm mole fraction: boron trifluoride partial pressure: || atm Total pressure in tank: atmarrow_forwardA 9.00 L tank at 1.5 °C is filled with 7.53 g of chlorine pentafluoride gas and 18.2 g of carbon monoxide gas. You can assume both gases behave as ideal gases under these conditions. Calculate the mole fraction of each gas. Round each of your answers to 3 significant digits. gas mole fraction ? chlorine pentafluoride carbon monoxidearrow_forward
- A 7.00 L tank at 6.54 °C is filled with 17.3 g of sulfur tetrafluoride gas and 17.7 g of chlorine pentafluoride gas. You can assume both gases behave as ideal gases under these conditions. Calculate the mole fraction and partial pressure of each gas, and the total pressure in the tank. Be sure your answers have the correct number of significant digits. mole fraction: х10 sulfur tetrafluoride partial pressure: | atm ? mole fraction: chlorine pentafluoride partial pressure: atm Total pressure in tank: atmarrow_forwardA sample of an unknown compound is vaporized at 100.°C . The gas produced has a volume of 1330.mL at a pressure of 1.00atm , and it weighs 4.52g . Assuming the gas behaves as an ideal gas under these conditions, calculate the molar mass of the compound. Be sure your answer has the correct number of significant digits.arrow_forwardA sample of an unknown compound is vaporized at 120. °C. The gas produced has a volume of 1850. mL at a pressure of 1.00 atm, and it weighs 1.95 g. Assuming the gas behaves as an ideal gas under these conditions, calculate the molar mass of the compound. Be sure your answer has the correct number of significant digits. g mol x10 Xarrow_forward
- A 9.00 L tank at 2.1 °C is filled with 7.71 g of boron trifluoride gas and 9.11 g of carbon dioxide gas. You can assume both gases behave as ideal gases under these conditions. Calculate the mole fraction and partial pressure of each gas, and the total pressure in the tank. Round each of your answers to 3 significant digits. mole fraction: boron trifluoride partial pressure: atm mole fraction: carbon dioxide partial pressure: atm Total pressure in tank: atmarrow_forwardSome N2 gas is mixed with some O2 gas, and the sketch below shows a representative sample of the mixture. The total pressure of the mixture is measured, and found to be 3.00 atm. key carbon hydrogen nitrogen sulfur oxygen chlorine Calculate the mole fraction and partial pressure of each gas in this mixture. Round your answers to 3 significant digits. You may assume each gas behaves as an ideal gas. gas mole fraction partial pressure x10 N₂ 02 atm ☐ ☑ atmarrow_forwardFor many purposes we can treat ammonia NH3 as an ideal gas at temperatures above its boiling point of −33.°C. Suppose the temperature of a sample of ammonia gas is lowered from 23.0°C to −19.0°C, and at the same time the pressure is changed. If the initial pressure was 6.3atm and the volume increased by 55.0%, what is the final pressure? Round your answer to the correct number of significant digits.arrow_forward
- A sample of an unknown compound is vaporized at 120. °C. The gas produced has a volume of 2130. mL at a pressure of 1.00 atm, and it weighs 6.27 g. Assuming the gas behaves as an ideal gas under these conditions, calculate the molar mass of the compound. Round your answer to 3 significant digits. g x10 molarrow_forwardA 5.00 L tank at 7.96 °C is filled with 10.8 g of chlorine pentafluoride gas and 4.88 g of dinitrogen difluoride gas. You can assume both gases behave as ideal gases under these conditions. Calculate the mole fraction and partial pressure of each gas, and the total pressure in the tank. Round each of your answers to 3 significant digits. ol. mole fraction: x10 chlorine pentafluoride Ar ? partial pressure: atm mole fraction: dinitrogen difluoride partial pressure: atm Total pressure in tank: atmarrow_forwardA sample of an unknown compound is vaporized at 150. °C. The gas produced has a volume of 2330. mL at a pressure of 1.00 atm, and it weighs 6.37 g. Assuming the gas behaves as an ideal gas under these conditions, calculate the molar mass of the compound. Round your answer to 3 significant digits. g mol x10 ×arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY