Question
Q11
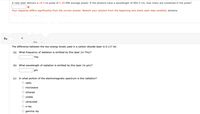
Transcribed Image Text:A ruby laser delivers a 18.5-ns pulse of 2.30-MW average power. If the photons have a wavelength of 694.3 nm, how many are contained in the pulse?
Your response differs significantly from the correct answer. Rework your solution from the beginning and check each step carefully. photons
T!
The difference between the two energy levels used in a carbon dioxide laser is 0.117 ev.
(a) What frequency of radiation is emitted by this laser (in THz)?
THz
(b) What wavelength of radiation is emitted by this laser (in um)?
um
(c) In what portion of the electromagnetic spectrum is this radiation?
O radio
O microwave
O infrared
O visible
O ultraviolet
O x-ray
O gamma ray
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Similar questions
- A block attached to a spring with a stiffness of 3.4 N/m. The relaxed length of the spring is 15 cm and its current length is 10 cm. What is the vector force that the spring exerts on the block? A. (-0.34, 0, 0) N B. (0.51, 0, 0) N C. (-0.51, 0, 0) N D. (0.17, 0, 0) N E. (-0.17, 0, 0) Narrow_forward3arrow_forwardQ11. Polar bears are strong swimmers and can swim as long as 10 hours without rest. If a polar bear swims with an average speed of 2.6 m/s, what is the total distance it can swim in 10.0 hours? Give your answer in km.arrow_forward
- E1. Suppose that a pancake recipe designed to feed five people calls for 310 grams of flour. How many grams of flour would you use if you wanted to reduce the recipe to only feed two people? {A: E2. Suppose that a cupcake recipe designed to produce 16 cupcakes calls for 240 grams of flour. How many grams of flour would you use if you wanted to make 20 cupcakes? E3. It is estimated that eight medium pizzas are about right to serve a physics club meeting of 32 students. How many pizzas would be required if the group, due to a conflicting math club meeting, was only going to have 20 students in attendance? A: E4. A child uses her hand to measure the width of a tabletop. If her hand has a width of 8 cm at its widest point, and she finds the tabletop to be 16.5 hands wide, what is the width of the tabletop in cm? In meters? Ε11. Area is found by multiplying the length of a surface times the width. If a floor measures 5.28 m², how many square centimeters does this represent? How many square…arrow_forwardQ3, Q,4 and Q5arrow_forward
arrow_back_ios
arrow_forward_ios