
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
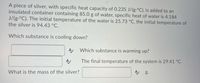
Transcribed Image Text:A piece of silver, with specific heat capacity of 0.235 J/(g.°C), is added to an
insulated container containing 85.0 g of water, specific heat of water is 4.184
J/(g.°C). The initial temperature of the water is 25.73 °C, the initial temperature of
the silver is 94.43 °C.
Which substance is cooling down?
A Which substance is warming up?
The final temperature of the system is 29.41 °C.
A g.
What is the mass of the silver?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Il Metro by T-Mobile 1:06 AM n 24% Question 15 of 35 Submit Specific Heat Capacity J/(g•°C) Metal Aluminum 0.90 Сopper 0.38 Iron 0.45 Magnesium 1.00 Silver 0.23 Tin 0.32 Titanium 0.52 A) Fe B) Sn C) Ag D) Cu Tap here or pull up for additional resourcesarrow_forwardYou wish to heat water to make coffee. How much heat (in joules) must be used to raise the temperature of 0.120 kg of tap water (enough for one cup of coffee) from 22°C to 96°C (near the ideal brewing temperature)? Assume the specific heat is that of pure water, 4.18 J/(g.°C). Heat = Jarrow_forwardWhen a solid dissolves in water, heat may be evolved or absorbed. The heat of dissolution (dissolving) can be determined using a coffee cup calorimeter. Thermometer Cardboard or In the laboratory a general chemistry student finds that when 3.38 g of CuCl,(s) are Styrofoam lid dissolved in 114.10 g of water, the temperature of the solution increases from 25.37 to 27.99 °C. The heat capacity of the calorimeter (sometimes referred to as the calorimeter constant) was determined in a separate experiment to be 1.60 J/°C. Nested Styrofoam cups Based on the student's observation, calculate the enthalpy of dissolution of CuCl2(s) in kJ/mol. - Reaction occurs in Assume the specific heat of the solution is equal to the specific heat of water. solution. AHdissolution kJ/mol kc Cengage Lnarrow_forward
- A 382−g aluminum engine part at an initial temperature of 18.76C absorbs 97.3 kJ of heat. What is the final temperature of the part (c of Al = 0.900 J/gK)?arrow_forwardA cubic piece of platinum metal (specific heat capacity = 0.1256 J/°C・g) at 200.0°C is dropped into 1.00 L of deuterium oxide ('heavy water,' specific heat capacity = 4.211 J/°C・g) at 25.5°C. The final temperature of the platinum and deuterium oxide mixture is 31.3°C. The density of platinum is 21.45 g/cm³ and the density of deuterium oxide is 1.11 g/mL. What is the edge length of the cube of platinum, in centimeters?arrow_forwardA 248-g aluminum engine part at an initial temperature of 16.04°C absorbs 66.3 kJ of heat. What is the final temperature of the part (c of Al = 0.900 J/g.K)? °Carrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY