
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
And which reaction is nitrogen reduced

Transcribed Image Text:A) NO2L9)+ Hho(1) → MNOg (an)
b) N,S) + 3Ha()→2NHる(の)
3H2 (9)>2NH3 9)
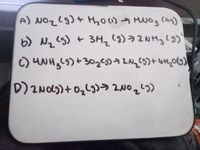
Transcribed Image Text:A) NOzlg)+ Hho() -→ HNO3 can)
6) Nq (S) + 3H2's)>2NH3(g)
D)2Nd)+0,L)う 2N025)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The following skeletal oxidation-reduction reaction occurs under acidic conditions. Write the balanced OXIDATION half reaction. CIO3+ H,S- CIO2 + S Products Reactants Submit Answer Retry Entire Group 9 more group attempts remainingarrow_forwardNNO(g) Incorrect : Narrow_forwardWrite a balanced half-reaction for the reduction of nitrate ion (NO, ) to gaseous nitrogen dioxide (NO,) in basic aqueous solution. Be sure to add physical state symbols where appropriate.arrow_forward
- Given the following two half reactions write a balanced oxidation-reduction reaction that occurs between sulfite and Ru3+ in acidic solution. Recall that a good way to proceed is to balance each half reaction in acidic solution, adjust one or both to have the same number of electrons gained as lost, and then add them together ...SO32- → SO42- and Ru3+ → Ru2+A) H2O + SO32- + Ru3+ → Ru2+ + SO42- + 2H+B) 6 H2O + 2 SO32- + Ru3+ → Ru2+ + 2 SO42- + 12 H+C) H2O + SO32- + 2 Ru3+ → 2 Ru2+ + SO42- + 2H+D) H2O + SO32- + 2 Ru3+ → 2 Ru2+ + SO42- + 2e- + 2H+arrow_forwardGive detailed Solution with explanation needed..don't give Handwritten answer..arrow_forwardWhat is the oxidation state of an individual sulfur atom in SO,2- Express the oxidation state numerically (e.g., +1). ?arrow_forward
- Using the Atoms, Oxygen, Hydrogen, and Electron (AOHE) method please help me balance the oxidation/reduction reactions I provided an example below on how to solve it using this method thank you so much! Directions: are to write the half reaction equation for the following and to state if the reaction is a reduction or oxidation reaction.arrow_forwardBalence the following half reation under acidic conditions: SO2 → SO42-arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY