
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Dd.25.
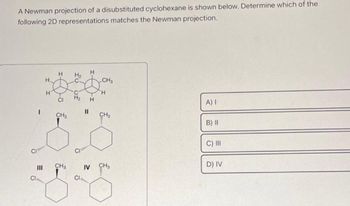
Transcribed Image Text:A Newman projection of a disubstituted cyclohexane is shown below. Determine which of the
following 2D representations matches the Newman projection.
I
|||
Cl
H.
H
H
CI
CH3
CH3
H₂
H
H₂ H
11
C/
IV
CH3
H
CH3
CH3
A) I
B) II
C) III
D) IV
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculate the amount of C2H6 (in grams) required to generate 2.5 g of CO2. 2C2H6(g) + 7O2(g) à 4CO2(g) + 6H2O(g) a.) 3.2 b.) 1.4 c.) 0.67 d.) 0.85arrow_forward5. a. What is % w/w when 15.5 grams of C6H12O6 dissolves in enough water to make 251 grams of solution? b. Determine mass of the solvent. 6.A What is % w/v when 15.5 ml of C₂H5OH (density = 0.785 g/ml) dissolves in 251 grams of water? B What is % w/w when 15.5 ml of C₂H5OH (density = 0.785 g/ml) dissolves in 251g of solution? CWhat is the molarity of solution, when 15.5 grams of C₂H5OH dissolves in enough water to make up 251 mL of solution? DCalculate ppb and ppm of the above solution 10. Show your calculation for preparation of 230.0 mL of 0.265 M sodium hydroxide solution from 0.850M sodium hydroxide.arrow_forwardIs the a( electrophillic or neucleophillic ) ( addition, elimination or substitute) reaction?arrow_forward
- Name each of the following organic compounds: i. i. CH; CH3 HC=C-CH;–CH-CH; CH;-CH-CH=CH-CH,CH,CH,CH; i. OH iv. CH3 CH,CH3arrow_forwardHow many grams of NH3 can be produced by mixing 14 g of N2 with 6.0 g of H2? Given: N2 + 3 H2 ------> 2 NH3 a.) 20g b.) 17g c.) 34g d.) 51garrow_forward22 23 24 25 2. Gaseous butane (CH, (CH,) CH, reacts with gaseous oxygen gas (0,) to produce gaseous carbon dioxide (CO,) and gaseous water (H,O). What is theoretical yield of carbon dioxide formed from the reaction of 5.81 g of butane and 19.8 g of oxygen gas? Round your answer to 3 significant figures. Ox10 Continue Submit Assig. 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Acce: MacBook Air 80 F1 DII DD F2 F3 F4 F5 F6 F7 F8 F9 F10 FILarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY