
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Please try to give type solution fast i will rate for sure
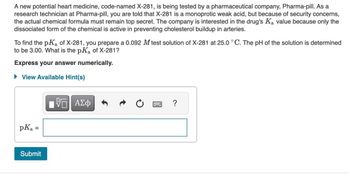
Transcribed Image Text:A new potential heart medicine, code-named X-281, is being tested by a pharmaceutical company, Pharma-pill. As a
research technician at Pharma-pill, you are told that X-281 is a monoprotic weak acid, but because of security concerns,
the actual chemical formula must remain top secret. The company is interested in the drug's Ka value because only the
dissociated form of the chemical is active in preventing cholesterol buildup in arteries.
To find the pK₂ of X-281, you prepare a 0.092 M test solution of X-281 at 25.0 °C. The pH of the solution is determined
to be 3.00. What is the pK₂ of X-281?
Express your answer numerically.
► View Available Hint(s)
pKa
=
Submit
——| ΑΣΦ
?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Balance each of the following reaction ___ C4H8 (g) + ___ O2 (g) à ___ CO2 (g) + ___ H2O (g)arrow_forwardPlease try to give type solution fast i will rate for surearrow_forwardIn chemistry class, a student conducted an investigation about factors affecting the solubility of copper (II) sulfate (CuSO4) in water. The data was recorded in the table below. Factors Trial 1 Trial 2 Trial 3 Stirring No No Yes Temperature 60°C 60°C 25°C Finely Crushed Powder Finely Crushed Powder Surface Area Large crystals Q1)Which trial should dissolve the slowest? Q2) How are the collisions between the CuS04 and water affected?arrow_forward
- Do the calculations to determine reaction order of both HCl and Na2S2O3 based on data givenarrow_forward! A C Escape Chemistry 106! Type here to search Final Exam Equation Sheet.pdf: X https://docs.google.com/forms/d/e/1FAIpQLSfdH4Cb3Jih0g_TXnrExG40h2E11 NuVCU4MS9 120% The Ksp values for four salts are shown in the picture. The solubility of THREE of * the salts can be directly compared without doing any math. Arrange the three salts in order of increasing solubility. Example: If the three salts that can be compared are the first three and they are already in order of increasing solubility, enter ABC. Solid A: Solid B: Solid C: Solid D: Your answer + Try again! A₂Br3 X₂F5 Y2Cl3 Z₂F3 3.4 x 10-² 7.8 x 10-5 1.9 x 10-16 6.4 x 10-8 Ⓒ↓|||| Barium sulfite (BaSO3) is a sparingly soluble salt. You need to dissolve as much * || a W 65°F Mostly cloudy J ABP 8:28 PM 5/14/2023arrow_forwardUsing the image attached for conclusion please make 1 paragraph - The cations present in cation mixture were... - The anions present in anions mixture were... - The cations present in unknown solution were... - The anions present in unknown solution were... Please please please answer as fast as possible pleasearrow_forward
- Need help : Information you can use: Volume vinegar used (mL) : 5.00 mL % from label : 5 % Molarity of NaOH : 0.0982 M _____________________________________________________ Inital volume NaOH (mL) Trial 1: 0.89 Trial 2: 0.41 Trial 3 :0.57 Final volume NaOH (mL) Trial 1:43.65 Trial 2: 43.49 Trial 3: 43.09 Volume NaOH used (mL) Trial 1: 42.76 Trial 2: 43.08 Trial 3: 42.52arrow_forwardCheck and correct the table and equationsarrow_forwardPls help ASAParrow_forward
- A sjc.cengagenow.com OWLV2 | Online teaching [References] Use the References to access important values if needed for this ques How many milliliters of 6.86 M hydrobromic acid solution should be used to prepare 4.50 L of 0.300 M HBr? mL Submit Answer Try Another Version 1 item attempt remaining Visitedarrow_forwardThe glassware which contacts the KMnO4 solution (the filter, suction flask and storage bottle), should all be pre-rinsed with a 10% HCl solution, AND THEN FULLY RINSED WITH DISTILLED WATER (VERY IMPORTANT STEP!!! – WHY??arrow_forwardQuantity Your Data 1. Grams of vinegar sample used for your titration 25.000 g 2. Initial Buret Reading of Sodium Hydroxide solution 10.00 mL 3. Final Buret Reading of Sodium Hydroxide solution 28.00 mL 4. Amount of Sodium Hydroxide Solution used to neutralize the vinegar sample 5. Concentration of NaOH in the NaOH solution 0.050 g/mL 6. Grams of NaOH used to neutralize the vinegar 7. Grams of acetic neutralized by the amount of NaOH 8. Percent acetic acid in the vinegar Hints: For #4, The amount of NaOH used is the difference between the starting buret value and the ending buret value. For #6, Once you calculate the amount of sodium hydroxide used, multiple that value by the concentration of NaOH in the NaOH solution. For #7, Multiply the value obtained in number 6 by the number 1.5. Remember, we learned that every 1 gram of NaOH neutralizes 1.5 grams of acetic acid. For #8, Divide the grams of acetic acid by the grams of vinegar sample and multiply this value by…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY