
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
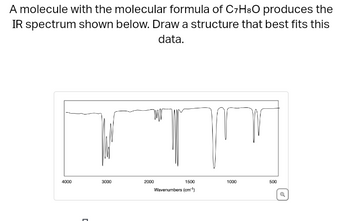
Transcribed Image Text:A molecule with the molecular formula of C7H8O produces the
IR spectrum shown below. Draw a structure that best fits this
data.
4000
D
3000
TO
2000
1500
Wavenumbers (cm'¹)
1000
500
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Select a single sp3–sp3 or sp3–sp2 bond from a linear portion of the molecule, identify it, and draw a Newman projection for it. Then draw a Newman projection for a 60 degree rotational isomer. List each rotational isomer as lower or higher energy. If your molecule is cyclic and has no linear portions, then draw the expected conformation of the cyclic portion of the molecule.arrow_forward7. Consider the C=O stretching data for the following cyclic ketones: cyclopropane 1813 cm³¹; cyclobutanone 1791 cm³¹; cyclopentanone 1740 cm³¹. (a) which ketone has the strongest bond? (b) where in the trend would you expect the C=O stretch of cyclopropenone? (Use the hybridization information in the same way we used hybridization for C-H stretching.)arrow_forwardWhich of the following conjugated systems would have the smallest HOMO-LUMO gap (i.e. largest λ-max of absorbance)? a) 1 b) 2 c) 3 d) 4arrow_forward
- A molecule produces two molecular ions with a m/z of 152 and 154 and a base peak with a m/z of 73. The IR and ¹H NMR spectra are shown below. Draw the structure that best fits this data. 4000 1H 11 10 3000 9 8 2000 7 1500 Wavenumbers (cm) + 6 5 2H 1000 2H 2 1 500 ppm of Q Qarrow_forwardAssign the correct constitutional isomer to this spectrum (C5H12O)arrow_forwardWhich of the following molecules would be expected to have absorbance in the IR range from a symmetric stretching vibration? SO2 CS2 CF4 CO2 CH4arrow_forward
- 48arrow_forwardThe absorption pattern in the UV/VIS region corresponds to (a) bond vibrations b) valance electron transitions =) molecular rotations E) nuclear spin 0000 a an IR spectrumarrow_forward22. Which of the following compounds gives an infrared Spectrum with peaks at 3300cm (sharp Peak) and 2150 cm (sharp Peak) ? H LH ₂ CH ₂ C = CH CH3C=CCH 3 2 1 HCCH H₂C A) I B)2 c) 3 04 CAZ H 9 CH₂arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY