
Chemistry & Chemical Reactivity
9th Edition
ISBN: 9781133949640
Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
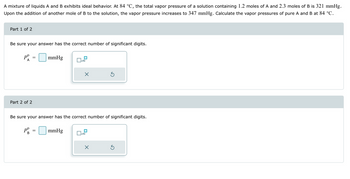
Transcribed Image Text:A mixture of liquids A and B exhibits ideal behavior. At 84 °C, the total vapor pressure of a solution containing 1.2 moles of A and 2.3 moles of B is 321 mmHg.
Upon the addition of another mole of B to the solution, the vapor pressure increases to 347 mmHg. Calculate the vapor pressures of pure A and B at 84 °C.
Part 1 of 2
Be sure your answer has the correct number of significant digits.
Pº
A
Part 2 of 2
=
mmHg
Ox x10
Pº = mmHg
B
X
Be sure your answer has the correct number of significant digits.
x10
Ś
X
Ś
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 70 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Two samples of sodium chloride solutions are brought to a boil on a stove. One of the solutions boils at 100.10C and the other at 100.15C. a Which of the solutions is more concentrated? b Which of the solutions would have a lower freezing point? c If you split the solution that boils at 100.1C into two portions, how would the boiling points of the samples compare? Which of the following statements do you agree with regarding the determination of your answer for part c? I. The question cannot be answered with certainty without knowing the volumes of each portion. II. Making the necessary assumption that the two samples have equal volumes, I was able to correctly answer the question. III. The volumes that the sample was split into are irrelevant when determining the correct answer.arrow_forward1. Vapor pressure: Arrange the following aqueous solutions in order of increasing vapor pressure at 25°C: 0.35 m C2H4(OH)2 (ethylene glycol, nonvolatile solute); 0.50 m sugar; 0.20 m KBr; and 0.20 m Na2SO4. C2H4(OH)2 < sugar < KBr < Na2SO4 Na2SO4 < sugar < KBr < C2H4(OH)2 sugar < C2H4(OH)2 < KBr < Na2SO4 KBr < sugar < Na2SO4 < C2H4(OH)2arrow_forwardGlycerin, C3H8O3, is a nonvolatile liquid. What is the vapor pressure of a solution made by adding 164 g glycerin to 338 mL H2O at 39.8C? The vapor pressure of pure water at 39.8C is 54.74 torr and its density is 0.992 g/cm3.arrow_forward
- A forensic chemist is given a white solid that is suspected of being pure cocaine (C17H21NO4, molar mass = 303.35 g/mol). She dissolves 1.22 0.01 g of the solid in 15.60 0.01 g benzene. The freezing point is lowered by 1.32 0.04C. a. What is the molar mass of the substance? Assuming that the percent uncertainty in the calculated molar mass is the same as the percent uncertainty in the temperature change, calculate the uncertainty in the molar mass. b. Could the chemist unequivocally state that the substance is cocaine? For example, is the uncertainty small enough to distinguish cocaine from codeine (C18H21NO3, molar mass = 299.36 g/mol)? c. Assuming that the absolute uncertainties in the measurements of temperature and mass remain unchanged, how could the chemist improve the precision of her results?arrow_forwardArrange 0.10 m aqueous solutions of the following solutes in order of decreasing freezing point and boiling point. (a) Al(ClO3)3(b) CH3OH (c) (NH4)2Cr2O7 (d) MgSO4arrow_forwardIce Cream A rock salt (NaCl), ice, and water mixture isused to cool milk and cream to make homemade icecream. How many grams of rock salt must be added towater to lower the freezing point by 10.0°C?arrow_forward
- Calculate the freezing point and normal boiling points of each of the following aqueous solutions. (a) 2.63 m acetic acid (b) 33.0 % by mass lactose, C12H22O11 (c) 32.15 mL of ethylene glycol, C2H6O2(d=1.113g/mL) in 624 mL of water (d=1.00g/mL)arrow_forwarda. Use the following data to calculate the enthalpy of hydration for calcium chloride and calcium iodide. Lattice Energy Hsoln CaCl2(s) 2247kj/mol 46kj/mol Cal2(s) 2059kj/mol 104kj/mol b. Based on your answers to part a, which ion, Cl or I, is more strongly attracted to water?arrow_forwardCalculate the freezing point of 525 g of water that contains 25.0 g of NaCl. Assume i, the vant Hoff factor, is 1.85 for NaCl.arrow_forward
- Which of the following salts, Li2SO4 or Cs2SO4, is expected to have the more exothermic enthalpy of hydration?arrow_forwardConsider two hypothetical pure substances, AB(s) and XY(s). When equal molar amounts of these substances are placed in separate 500-mL samples of water, they undergo the following reactions: AB(s)A+(aq)+B(aq)XY(s)XY(aq) a Which solution would you expect to have the lower boiling point? Why? b Would you expect the vapor pressures of the two solutions to be equal? If not, which one would you expect to have the higher vapor pressure? c Describe a procedure that would make the two solutions have the same boiling point. d If you took 250 mL of the AB(aq) solution prepared above, would it have the same boiling point as the original solution? Be sure to explain your answer. e The container of XY(aq) is left out on the bench top for several days, which allows some of the water to evaporate from the solution. How would the melting point of this solution compare to the melting point of the original solution?arrow_forwardThe solubility of NaCl in water at 100 C is 39.1 g/100. g of water Calculate the boiling point of this solution. (Assume i = 1.85 for NaCl.)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning