
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
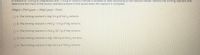
Transcribed Image Text:A mixture of 125.0 g of magnesium and 175.0 g of iron(lll) chloride is allowed to react according to the reaction below. Identify the limiting reactant and
determine the mass of the excess reactant present in the vessel when the reaction is complete.
BMg(s) + 2FeCl3(aq)→ 3MGCI2(aq) + 2Fe(s)
O A. The limiting reactant is Mg: 9.4 g of FeCl3 remains.
O B. The limiting reactant is FeCl3; 17.0 g of Mg remains.
OC. The limiting reactant is FeCl3: 85.7 g of Mg remains.
O D. The limiting reactant is Mg: 109.0 g of FeClg remains.
OE. The limiting reactant is Mg: 64.5 g of FeClg remains.
Expert Solution
arrow_forward
Step 1
Given,
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Chlorine gas can be prepared in the laboratory by the reaction of hydrochloric acid with manganese (IV) oxide. 4 HCl(aq) + MnO₂ (s) → MnCl₂ (aq) + 2 H₂O(l) + Cl₂ (g) A sample of 42.5 g MnO₂ is added to a solution containing 50.9 g HCl. What is the limiting reactant? O MnO₂ HC1 What is the theoretical yield of Cl₂? theoretical yield: If the yield of the reaction is 84.1%, what is the actual yield of chlorine? actual yield: g Cl₂ g Cl₂arrow_forwardWhat mass of precipitate (in g) is formed when 80.0 mL of 0.500 M AlCl₃ reacts with excess AgNO₃ in the following chemical reaction? AlCl₃(aq) + 3 AgNO₃(aq) → 3 AgCl(s) + Al(NO₃)₃(aq)arrow_forwardDetermine the maximum (theoretical) and actual yield of CaCO3, in grams, that can be produced from the precipitation reaction given 105.99 mol of Na2CO3. Na2CO3 (aq) + CaCl2 ⋅2H2O → CaCO3 (s) + 2NaCl(aq) + 2H2O(aq)arrow_forward
- A sample of 7.89 g of Mg(OH)2 is added to 24.6 mL of 0.190 M HNO3. Which is the limiting reactant in the reaction? a) Mg(NO3)2 b) Mg(OH)2 c) HNO3 d) H2Oarrow_forwardWhat mass of precipitate (in g) is formed when 77.9 mL of 0.500 M FeCl₃ reacts with excess AgNO₃ in the following chemical reaction? FeCl₃(aq) + 3 AgNO₃(aq) → 3 AgCl(s) + Fe(NO₃)₃(aq)arrow_forwardHow many moles of Cl₂ react with 21.6 g NaOH for this reaction? 2Cl₂ (g) + 4NaOH (aq) → 3NaCl (aq) + NaClO₂ (aq) + 2H₂O (l)arrow_forward
- Iron(III) oxide reacts with carbon monoxide according to the equation:Fe2O3(s)+3CO(g)→2Fe(s)+3CO2(g)A reaction mixture initially contains 22.35 g Fe2O3Fe2O3 and 15.54 g CO Once the reaction has occurred as completely as possible, what mass (in g) of the excess reactant is left?arrow_forwardA piece of magnesium with a mass of 0.0501 grams was placed into a solution containing an excess of hydrochloric acid. The reaction proceeds according the equation below. Mg(s) + 2HCl(aq) ⟶ MgCl2(aq) + H2(g) Assuming the reaction goes to completion, how many electrons are transferred in this reaction?arrow_forwardSuppose 0.0350 g Mg is reacted with 10.00 mL of 6 M HCI to produce aqueous magnesium chloride and hydrogen gas. Mg(s) + 2HC1(aq) → MgCl2(aq) +H2(g) What is the limiting reactant in this reaction? Select one: Magnesium metal Hydrochloric acid Magnesium chloride Hydrogen gasarrow_forward
- What mass of precipitate (in g) is formed when 45.5 mL of 0.300 M Na₃PO₄ reacts with 58.5 mL of 0.200 M CrCl₃ in the following chemical reaction? Na₃PO₄(aq) + CrCl₃(aq) → CrPO₄(s) + 3 NaCl(aq)arrow_forwardWhat mass of precipitate (in g) is formed when 80.0 mL of 0.50 M AlCl₃ reacts with excess AgNO₃ in the following chemical reaction? AlCl₃(aq) + 3 AgNO₃(aq) → 3 AgCl(s) + Al(NO₃)₃(aq)arrow_forwardChlorine gas can be prepared in the laboratory by the reaction of hydrochloric acid with manganese(IV) oxide. 4HCl(aq)+MnO2(s)⟶MnCl2(aq)+2H2O(l)+Cl2(g) A sample of 36.7 g MnO2 is added to a solution containing 43.7 g HCl. What is the limiting reactant? HCl MnO2 What is the theoretical yield of Cl2? theoretical yield:_____g Cl2g If the yield of the reaction is 74.9%, what is the actual yield of chlorine? actual yield:_____g Cl2arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY