
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
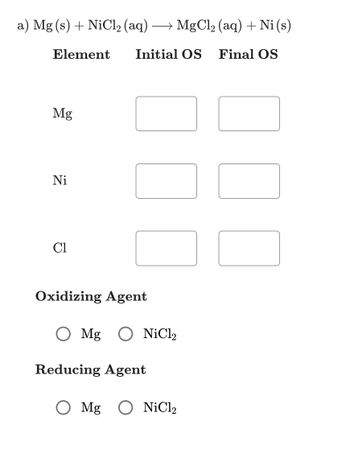
Transcribed Image Text:a) Mg(s) + NiCl₂ (aq) +MgCl,(aq) +Ni(s)
Element
Initial OS Final OS
Mg
Ni
Cl
0
Oxidizing Agent
O Mg O NiCl₂
Reducing Agent
O Mg O NiCl₂
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Chemistry Consider the following redox reactions assuming they occur in a basic solution. (a) SO, (aq) + Cu(OH), (s) →SO, (aq) + Cu(OH)(s) MnO₂ (s) (b) O₂(g) + Mn(OH)₂ (s) → (c) NO, (aq) + H₂(g) →→→ NO(g) (d) Al(s) + Cro, (aq) AK(OH), (s) + Cr(OH), (aq) Which of the following presents a wrong balanced equation? Show your work on your worksheet. 2Cu(OH), (s) +SO, (aq) →→2 Cu(OH)(s) + SO₂³(aq) + H₂O(l) 2Mn(OH)₂ (s) + O₂(g) →→→ 2MnO₂ (s) + 2H₂O(l) (b) (c) 2NO, (aq) + 2(H* +OH) + 3H₂(g) → 2NO(g) + 4H₂O(l) (d) CO₂ (aq) + Al(s) +H*(aq) + 2H₂O(1)→→ C(OH), (aq) + Al(OH),(s)arrow_forwardBalance the equation and give the coefficient of the question mark (?). KOH + H3PO, → K3PO, + H20 (x)KOH + (?)H,PO, (x)K,PO, + (x)H,0 O 4 3,arrow_forwardPls help ASAP. Pls help on both PLSarrow_forward
- Assigning oxidation numbers Some chemical reactants are listed in the table below. Complete the table by filling in the oxidation state of the highlighted atom oxidation state of species highlighted atom VO(s) Cu (aq) %3Darrow_forward6. The following reaction forms a white precipitate. Is this a redox reaction? AgNO3(aq) + HCl(aq) → HNO3(aq) + AgCl(s)arrow_forwardStep by step please. also have to answer A-Darrow_forward
- educed. If it is not, select No and leave the following boxes blank. Express your answers as a chemical formulas. Omit st Sc(s) + 3Ag (ag) 3 Ag(s) + Sc+ (aq) a. Redox? Oxidizing Agent Reducing Agent Substance Oxidized Substance Reduced b. HI(g) + NH3 (9) → NH,I(s) Oxidizing Agent Redox? Reducing Agent Substance Oxidized Substance Reduced SiBr. (1) + 2H2 0(1) → 4HB1(aq) + SiO2 (s) с. Oxidizing Agent Redox? Reducing Agent Substance Oxidized Substance Reducedarrow_forwardWhat is(are) the classification(s) of the reaction below? (Choose all that applies.) AGNO3(aq) + KCI(aq) → AgCI(s) + KNO3(aq) addition reaction single displacement reaction combustion reaction double displacement reaction acid-base neutralization reaction gas-forming reaction O precipitation reaction redox reaction decomposition reactionarrow_forwardFor each chemical reaction listed in the table below, decide whether the highlighted atom is being oxidized or r highlighted atom is being... reaction neither oxidized nor oxidized reduced reduced olo Cu SO 4(aq)+Zn(s) → ZnSO 4(aq)+Cu(s) 4 HF (9)+ SiO,(s) → SiF 4(9)+2 H,O(9) C(s)+O2(9) → CO,(9) N2(9)+3 H2(9) → 2 NH3(9)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY