
Introductory Chemistry: A Foundation
9th Edition
ISBN: 9781337399425
Author: Steven S. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:SC
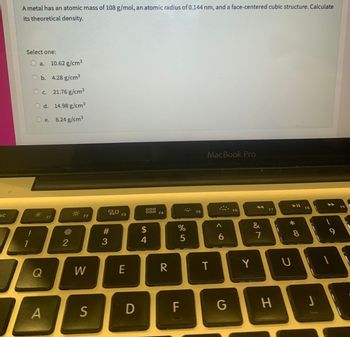
A metal has an atomic mass of 108 g/mol, an atomic radius of 0.144 nm, and a face-centered cubic structure. Calculate
its theoretical density.
Select one:
!
1
a. 10.62 g/cm³
b. 4.28 g/cm³
O c. 21.76 g/cm³
d.
14.98 g/cm³
8.24 g/cm³
Q
A
e.
F1
2
F2
W
S
80
#3
F3
E
D
000
$
54
F4
R
%
5
F
F5
MacBook Pro
T
A
6
G
F6
Y
&
7
F7
H
► 11
* 00
8
F8
UI
J
9
F9
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Metallic barium has a body-centered cubic structure (all atoms at the lattice points) and a density of 3.51 g/cm3. Assume barium atoms to be spheres. The spheres in a body-centered array occupy 68.0% of the total space. Find the atomic radius of barium. (See Problem 11.93.)arrow_forwardAn amorphous solid can sometimes be converted to a crystalline solid by a process called annealing. Annealing consists of heating the substance to a temperature just below the melting point of the crystalline form and then cooling it slowly. Explain why this process helps produce a crystalline solid.arrow_forwardSubstance B is hard, does not conduct electricity, and melts at 1200 C. Substance B is likely a(n): (a) ionic solid (b) metallic solid (c) molecular solid (d) covalent network solidarrow_forward
- Describe the unit cell of lithium (see Figure).arrow_forwardSubstance A is shiny, conducts electricity well, and melts at 975 C. Substance A is likely a(n): (a) ionic solid (b) metallic solid (c) molecular solid (d) covalent network solidarrow_forwardMnO has either the NaCI type structure or the CsCI type structure (see Exercise 69). The edge length of the MnO unit cell is 4.47 10-8 cm and the density of MnO is 5.28 g/cm3. a. Does MnO crystallize in the NaCl or the CsCl type structure? b. Assuming that the ionic radius of oxygen is 140. pm, estimate the ionic radius of manganese.arrow_forward
- The CsCl structure is a simple cubic array of chloride ions with a cesium ion at the center of each cubic array (see Exercise 69). Given that the density of cesium chloride is 3.97 g/cm3, and assuming that the chloride and cesium ions touch along the body diagonal of the cubic unit cell, calculate the distance between the centers of adjacent Cs+ and Cl ions in the solid. Compare this value with the expected distance based on the sizes of the ions. The ionic radius of Cs+ is 169 pm, and the ionic radius of Cl is 181 pm.arrow_forward8.98 If you know the density of material and the length of the edge of its cubic Iattice, how would you determine if it is face-centered cubic, body-centered cubic, or simple cubic Would you have to look up any information?arrow_forwardNaH crystallizes with the same ciystal structure as NaCl. The edge length of the cubic unit cell of NaH is 4.880. (a) Calculate the ionic radius of H. (The ionic radius of Li+ is 0.0.95 .) (b) Calculate the density of NaH.arrow_forward
- 8.16 Iridium forms a face-centered cubic lattice, and an iridium atom is 271.4 pm in diameter. Calculate the density of iridium.arrow_forwardPhase diagrams for materials that have allotropes can be more complicated than those shown in the chapter. Use the phase diagram for carbon given here to answer the following questions. (a) How many triple points are present and what phases are in equilibrium for each? (b) Is there a single point where all four phases are in equilibrium? (c) Which is more stable at high pressures, diamond or graphite? (d) Which is the stable phase of carbon at room temperature and 1 atmosphere pressure?arrow_forwardThe radius of tungsten is 137 pm and the density is 19.3 g/cm3. Does elemental tungsten have a face-centered cubic structure or a body-centered cubic structure?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning