
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question

Transcribed Image Text:The crystalline density of polypropylene is 1.121 g/cm³, and its amorphous density is 0.906 g/cm³. What is the weight percent of the structure that is crystalline in a
polypropylene that has a density of 1.054 g/cm³?
Select one:
a. 65 %
b. 73 %
c. 1.36 %
d. 13.6 %
Expert Solution
arrow_forward
Step 1
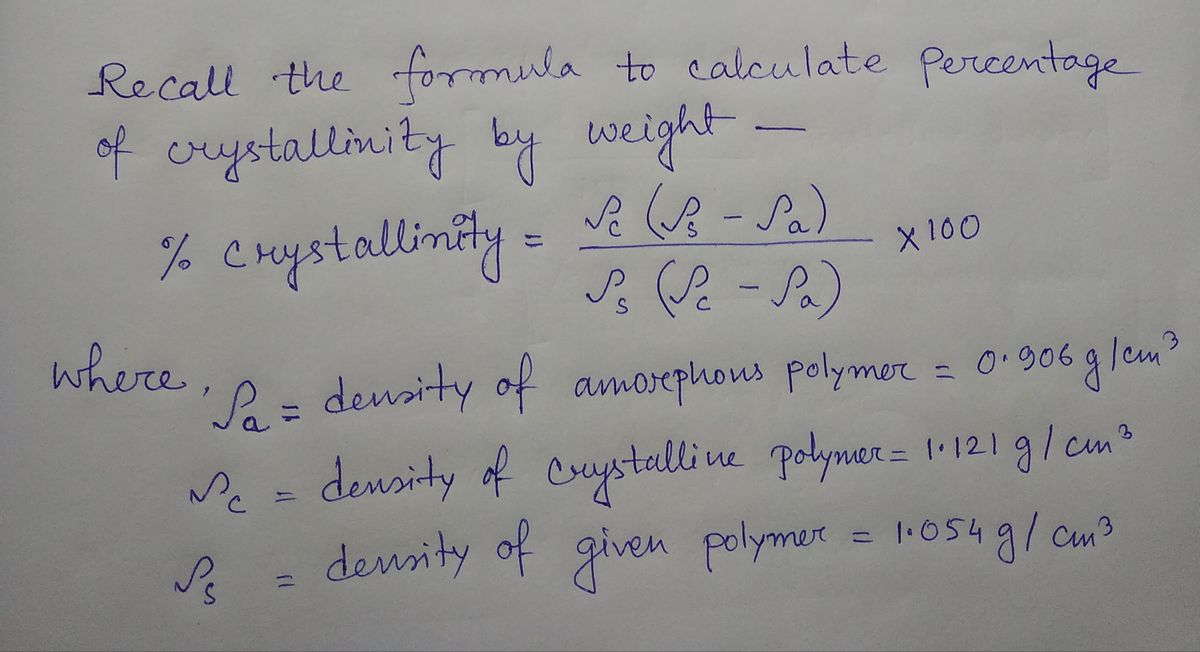
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Nickel crystallizes in face center cubic unit cell. What is the atomic radius of nickel if the density of the metal is 18.0 g/cm3? A. 1.40 x 10-8 cm B. 8.15 x10-9 cm C. 2.79 x 10-8 cm D. 1.21 x 10-8 cm E. 9.80 x 10-9 cmarrow_forwardHow many lattice points are there in a single repeating cell of an FCC crystalstructure? How many in a repeating cell of an HCP structure?a. FCC: 14 lattice points, HCP: 17 lattice pointsb. FCC: 8 lattice points, HCP: 5 lattice pointsc. FCC: 4 lattice points, HCP: 6 lattice pointsd. FCC: 4 lattice points, HCP: 5 lattice pointsarrow_forward25. Which statement about crystal structure is false? a. Rhombic, triclinic and cubic are examples of crystal structures. b. Amorphous substances have no organized crystal structure. c. Polymorphous substances have more than one crystal structure. d. Isomorphous substances have only one crystal structure. e. In a cubic lattice, four other cells share an atom lying at the corner of the unit cell equally.arrow_forward
- Indicate if each solid forms an n-type or a p-type semiconductor.a. germanium doped with galliumb. silicon doped with arsenicarrow_forward23. Tin crystallizes in a face centered cubic arrangement. The atomic radius of a tin atom is 1.41 x 10-8 cm. What is the volume of a unit cell of Sn, in cubic centimeters? A) 7.93 x 10-24 B) 6.34 x 10-23 C) 2.80 x 10-24 D) 2.24 x 10-23arrow_forwardOsmium tetraoxide, OsO4 is a molecular crystal. Which of the following general properties would you NOT expect it to possess: a. Being a poor conductor of electricity b. Having a soft rather than a brittle crystals c. Having a melting point around 40 degrees C d. Having a melting point of 1600 degrees Carrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY