
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
please detail solution given
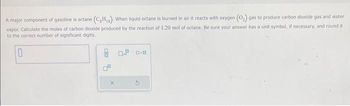
Transcribed Image Text:A major component of gasoline is octane (C₂H₁). When liquid octane is burned in air it reacts with oxygen (O₂) gas to produce carbon dioxide gas and water
vapor, Calculate the moles of carbon dioxide produced by the reaction of 1.20 mol of octane. Be sure your answer has a unit symbol, if necessary, and round it
to the correct number of significant digits.
0
X
0.9 0.0
G
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A common colligative property is that a solute will... Raise the boiling point none of these Raise the vapor pressure Raise the freezing point all of thesearrow_forwardIf i have 0.09079M HCL concentration solution & .09731M NaOH concentration solution What amount of NaOH would you expect to be necessary to neutralize 10.00mL of the HCL?arrow_forwardPlease give an equation to show why boiling water deviate from the neutral pH.arrow_forward
- Simulation of Vapour Pressure 1. Record the temperature and then the vapour pressure for each substance. Temperature = °C 2. Indicate the type(s) of intermolecular force acting between the molecules for each substance. methanol CH3OH ethanol CH3CH2OH propan-1-ol CHзCH:CH2ОH Vapour pressure: Vapour pressure: Vapour pressure: O Dispersion Dispersion O Dispersion Dipole/dipole Dipole/dipole Dipole/dipole Hydrogen bonding Hydrogen bonding Hydrogen bonding Substance with highest normal boiling point: Briefly discuss the trend in vapour pressure for these substances in terms of intermolecular forces: CI OH 2-chloropropanc H,C-C- CH3 | propan-2-ol H,c-с-сн, H;C-C- CH3 propanone H. H Vapour pressure: Vapour pressure: Vapour pressure: Dispersion Dipole/dipole Dispersion Dispersion Dipole/dipole Dipole/dipole Hydrogen bonding Hydrogen bonding Hydrogen bonding Substance with highest normal boiling point: Briefly discuss the trend in vapour pressure for these substances in terms of intermolecular…arrow_forwardWhich one is it?arrow_forwardIn the Flint Water Crisis, trihalomethanes were produced in the new water supply due to the mixing of ? with ? water.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY