
Human Anatomy & Physiology (11th Edition)
11th Edition
ISBN: 9780134580999
Author: Elaine N. Marieb, Katja N. Hoehn
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Question
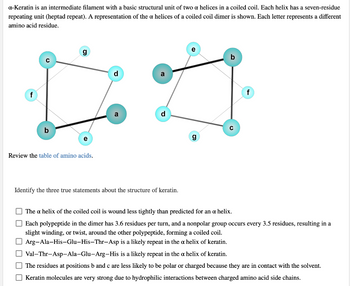
Transcribed Image Text:α-Keratin is an intermediate filament with a basic structural unit of two a helices in a coiled coil. Each helix has a seven-residue
repeating unit (heptad repeat). A representation of the a helices of a coiled coil dimer is shown. Each letter represents a different
amino acid residue.
f
g
d
e
Review the table of amino acids.
a
d
g
f
Identify the three true statements about the structure of keratin.
☐ The a helix of the coiled coil is wound less tightly than predicted for an α helix.
☐ Each polypeptide in the dimer has 3.6 residues per turn, and a nonpolar group occurs every 3.5 residues, resulting in a
slight winding, or twist, around the other polypeptide, forming a coiled coil.
Arg-Ala-His-Glu-His-Thr-Asp is a likely repeat in the a helix of keratin.
☐ Val-Thr-Asp-Ala-Glu-Arg-His is a likely repeat in the a helix of keratin.
☐ The residues at positions b and c are less likely to be polar or charged because they are in contact with the solvent.
☐ Keratin molecules are very strong due to hydrophilic interactions between charged amino acid side chains.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- b) It's said that secondary structures form because of intra- and intermolecular hydrogen bonding involving the peptide bond. Describe what is going on to further stabilize secondary structure through these interactions.arrow_forwardIdentify and encircle the peptide bonds in this polypeptide (Asp-Sec-Leu-Cys-Glu).arrow_forwardAssume you have a long polypeptide chain of Gly-Val dipeptides linked end to end. Explain the likely tertiary structure of this polypeptide chain. For example, is the chain likely to form an alpha helix, a beta sheet, or some other structure? Is the structure likely to display lots of folding, or very little folding? Can you make any other predictions about the tertiary structure? Explain your reasoning in a well-developed paragraph.arrow_forward
- "Potassium Bromate (KBr03) is added to flour as a softener that strengthens the texture of bread. It can do this by forming disulfide bonds between two cysteine amino acids in a reaction similar to the chemical equation given below. The function of KBr03 is to remove the 2 electrons needed to form the disulfide bond as shown in the figure below. Each of the cysteine amino acids will be in a protein chain, so the reaction binds the two chains together, giving the dough cohesion. However, potassium bromate is a proven carcinogen and is only allowed in foods in the United States because bakers will add the right amount and correct It has been assumed to meet the temperature and cooking time conditions. " KBRO, + C,H¸NO,SH → KBr + H,O+C,H„N,O,S, Balance the reaction of potassium bromate (KBr03) with the cysteine molecule (C3H6 NO2 SH) by converting it into the reduced step matrix using the additive matrix method. (Kis the symbol of potassium in the question) (Homogeneous linear equation…arrow_forwardWhich of the following amino acids would you least expect to find on the surface of a water-soluble protein? Explain I Serine Glutamat Lysime Threoninearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON
Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education,
Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education, Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company
Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co.
Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co. Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education
Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education

Human Anatomy & Physiology (11th Edition)
Biology
ISBN:9780134580999
Author:Elaine N. Marieb, Katja N. Hoehn
Publisher:PEARSON

Biology 2e
Biology
ISBN:9781947172517
Author:Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:OpenStax

Anatomy & Physiology
Biology
ISBN:9781259398629
Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa Stouter
Publisher:Mcgraw Hill Education,

Molecular Biology of the Cell (Sixth Edition)
Biology
ISBN:9780815344322
Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter Walter
Publisher:W. W. Norton & Company

Laboratory Manual For Human Anatomy & Physiology
Biology
ISBN:9781260159363
Author:Martin, Terry R., Prentice-craver, Cynthia
Publisher:McGraw-Hill Publishing Co.

Inquiry Into Life (16th Edition)
Biology
ISBN:9781260231700
Author:Sylvia S. Mader, Michael Windelspecht
Publisher:McGraw Hill Education