
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
b) It's said that secondary structures form because of intra- and intermolecular hydrogen bonding involving
the peptide bond. Describe what is going on to further stabilize secondary structure through these interactions.
Transcribed Image Text:A
סגי
Z-I
N
HHR
H O RH
O
0=
N
O H
R
..I-Z
FO:::
H O
FO
I-
HR
RH
I-Z
0=
R
=
Z-I
N
Z-I
O H
H O
HR
RH
B
R
m
vin
D
O
H
I
N
R
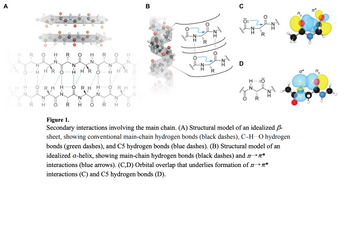
Figure 1.
Secondary interactions involving the main chain. (A) Structural model of an idealized -
sheet, showing conventional main-chain hydrogen bonds (black dashes), C-HO hydrogen
bonds (green dashes), and C5 hydrogen bonds (blue dashes). (B) Structural model of an
idealized a-helix, showing main-chain hydrogen bonds (black dashes) and n*
interactions (blue arrows). (C,D) Orbital overlap that underlies formation of n*
interactions (C) and C5 hydrogen bonds (D).
n
0*
TT*
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Similar questions
- 1)Explain 3 benefits of proteins forming higher oligomeric states. 2) Why are peptide bonds planar?arrow_forwardCompare and contrast important features of the peptide backbone of proteins and the phosphate backbone of nucleic acids by 1. Number of bonds in the backbone, 2. Bond order and amount of rotation, 3. Charge of backbone, and 4. Types of interactions backbone may form with other molecules and ions (info about interactions and ligands)arrow_forwardDraw the structure of the following peptide as it would be at pH = 7. Does the molecule have a net charge at that pH? ExplainMet-Ser-Glu-Ala-Lys-Lys-Gluarrow_forward
- The peptide bond is a stronger bond than the ester bond. What structural feature of the peptide bond gives it additional bond strength?arrow_forwardWhich of the following most directly applies to the formation of the secondary structures of proteins? A B с D formation of ionic bonds between the R groups of two polypeptides formation of nonpolar interactions between two R groups on the same polypeptide formation of covalent bonds via dehydration reactions between two amino acids formation of hydrogen bonds between between amino and carboxyl groupsarrow_forwardSelect A letter for each partarrow_forward
- Refer to the structure of an oligopeptide below: OH но. NH SH Taking note of the component residues, would such a structure as it is drawn above be possible at any pH value? (Yes or No)?arrow_forwardHaving peptides arranged in a beta sheet would be an example of a secondary structure A) True B) Falsearrow_forwardDraw the structure of Lys and Ala linked by an isopeptide bond.arrow_forward
- The following figure is a diagram (cartoon or caricature) of the structure of a protein. What types of secondary structure are observed in the molecule? Is it a globular protein or a fibrous one?arrow_forwardDisulfide bonds help to stabilize the three-dimensional structure of proteins. What amino acids are involved in the formation of disulfide bonds? Does the formation of a disulfide bond increase or decrease entropy (ΔS)?arrow_forwardWhat are the similarities and differences of intermolecular interactions that stabilize secondary versus tertiary structure? Think about types of interactions, side-chain versus backbone interactions, and proximity of the residues involved. The molecule considered is a protein: pancreatic amylase.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON