
Chemistry: Principles and Reactions
8th Edition
ISBN: 9781305079373
Author: William L. Masterton, Cecile N. Hurley
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Identify conjugate acids and bases in the following pairs
of substances:
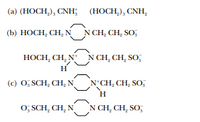
Transcribed Image Text:(a) (HOCH,), CNH;
(HOCH,), CNH,
(b) HOCH, CH, N N CH, CH, SO,
HOCH, CH, NN CH, CH, SO;
H
(e) O; SCH, CH, NN CH, CH, SO;
H
0, SCH, CH, NN CH, CH, SO,
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Rank the elements or compounds in the table below in decreasing order of their boiling points. That is, choose 1 next to the substance with the highest boiling point, choose 2 next to the substance with the next highest boiling point, and so on, substance D HI chemical symbol, chemical formula or Lewis structure H HH ||| HHH Ar H I Ag -0. H H :O: H |||| HICIC-CIH H boiling point (Choose one) (Choose one) (Choose one) (Choose one)arrow_forwardO-H H CH,OH (b) CH, CH, NH, H--o=C (Ь) 0-H--O (а) CH, CH, CH, CH, CH H-C CH, HC CH, | (d) CH, (CH,)4–NH, (a) CH, (d) (с) CH, H,C CH, CH CH, CH,COOH (Б) H Harrow_forwardH. NaBH3CN 1) xs CH3I 2) Ag.O/H,О/heatarrow_forward
- t- U- S- 요 s O NHẠCH3, H® NHE+₂, HⓇ CN Ⓒ ΝΗ, Ηarrow_forwardWhere c-h: 413, c=c: 614, o-h:467, c-c: 347, c-o:358arrow_forwardRank the elements or compounds in the table below in decreasing order of their boiling points. That is, choose 1 next to the substance with the highest boiling point, choose 2 next to the substance with the next highest boiling point, and so on. substance A B с D H :0: H | || | H с C -C-H 174 H H H chemical symbol, chemical formula or Lewis structure H H H | | C I 1 I H H H (II | Ag - Ar - :O: -O-H boiling point (Choose one) (Choose one) ✓ (Choose one) (Choose one) ✓arrow_forward
- 62arrow_forwardOCH3 NH₂ CH3- OH 1. CH₂MgBr 2. H3O+ NH AICI 3 CI .&M H ze bes AICI3 1. excess CH3l 2. Ag₂O, H₂O 3. heat heat 1. Br2, PBr3 2. H₂O Hg, Zn, HCI 1. KOH 2. CI 3. KOH, H₂O, heat CH3 blank 1 blank 2 blank 3 blank 4 blank 5 blank 6 blank 7 blank 8 blank 9 1. KOCH3 2. H₂O, H+, heat blank 10 O-+K OH OH CH3CH₂Br NH,NH2, KOH H+ H₂, Pd DMSO, (COCI) 2 Et3N NaNH, NH3 Ag(NH3)2* KOH, H₂O KOH, heat PPh3 excess CH3OH H+ blank 11 blank 12 blank 13 blank 15 blank 14 blank 16 blank 17 blank 18 blank 19arrow_forwardplease. part Darrow_forward
- Rank the elements or compounds in the table below in decreasing order of their boiling points. That is, choose 1 next to the substance with the highest boiling point, choose 2 next to the substance with the next highest boiling point, and so on. chemical symbol, substance chemical formula boiling point or Lewis structure H H H A H – C C - C -0 H (Choose one) ▼ | H H Ag (Choose one) В Ar |(Choose one) - H :0: H Н— С — С С — Н (Choose one) v H. ? :0 : I - O - Tarrow_forwardThe table below contains some mean bond energy data: Bond C-C 348 H-H N=N Mean bond energy /kJ mol| 436 944 (i) Balance the equation below for the formation of one mole of ammonia, NH3, from its elements. N2 + H2 NH3 AH = -38 kJmol" (ii)Use the data in the table above to calculate the bond energy of N - H bond in NH3 in the reaction given in (1) above. Comment on why the value obtained is referred to as 'mean bond enthalpy (ii) Use the equation below and data from the table to calculate a value for the C=C bond energy in ethene. Bond H-H C-H C-C 348 Mean bond energy /kJ mol 436 418 нн нн С—С + Н Нэнс- с н AH = -136 kJ mol нн н Ethene Ethanearrow_forward8arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Physical Chemistry
Chemistry
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Wadsworth Cengage Learning,

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning