
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
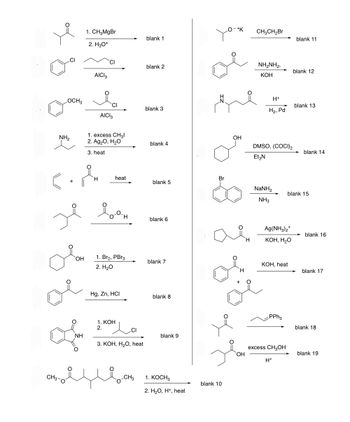
Transcribed Image Text:OCH3
NH₂
CH3-
OH
1. CH₂MgBr
2. H3O+
NH
AICI 3
CI
.&M
H
ze bes
AICI3
1. excess CH3l
2. Ag₂O, H₂O
3. heat
heat
1. Br2, PBr3
2. H₂O
Hg, Zn, HCI
1. KOH
2.
CI
3. KOH, H₂O, heat
CH3
blank 1
blank 2
blank 3
blank 4
blank 5
blank 6
blank 7
blank 8
blank 9
1. KOCH3
2. H₂O, H+, heat
blank 10
O-+K
OH
OH
CH3CH₂Br
NH,NH2,
KOH
H+
H₂, Pd
DMSO, (COCI) 2
Et3N
NaNH,
NH3
Ag(NH3)2*
KOH, H₂O
KOH, heat
PPh3
excess CH3OH
H+
blank 11
blank 12
blank 13
blank 15
blank 14
blank 16
blank 17
blank 18
blank 19
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Part 1 of 2 Determine the pH of a 0.30 M NH3 solution. Be sure your answer has the correct number of significant digits. -5 Note: K for NH₂ is 1.78 × 10¯ pH = x10 X Sarrow_forwardWhat volume (in L) of 1.60 M Na3PO4 would be required to obtain 0.870 moles of Na+ ions?arrow_forwardb if [ОH ] %3D7.6 х 10 4 M then РОН =arrow_forward
- Calculate the molarity of each of these solutions.a. A 5.623-g sample of NaHCO3 is dissolved in enough water to make 250.0 mL of solution. [3]b. A 184.6-mg sample of K2Cr2O7 is dissolved in enough water to make 500.0 mL of solution. [3]A solution of ethanol (C2H5OH) in water is prepared by dis-solving 75.0 mL of ethanol (density = 0.79 g/cm3) in enough water to make 250.0 mL of solution. What is the molarity of the ethanol in this solutionarrow_forward1. Calculate the number of moles in these quantities:(a) 25.0 g KNO3 (c) 5.4 x 102 g (NH4)2C2O4(b) 56 millimol NaOH (d) 16.8 mL H2SO4 solution (d = 1.727 g/mL, 80.0% H2SO4 by mass)arrow_forward2 Na + Cl2 --> 2 NaCl What is the mole ratio of sodium to sodium chloride? a. 2:1 b. 2:2 c. 1:2arrow_forward
- [1pt] A 0.86 M HCl solution is available in the laboratory. How much DI water is needed to prepare 15.0 mL of a 0.18 M HCI solution?arrow_forwardWhen you dissolve KCl in water, does the reaction give off heat or absorb heat? Is the dissolution of KCl in water endothermic or exothermic?arrow_forward#25y of a 23% by mass magnesium chloride (MW= 95.21 g/mole) solution is reacted with 125.0 mL da125M solution of silver nitrate (MW= 169.87g/mole) determine the mass of silver chloride (MW = 143.32 p/mole) producedarrow_forward
- a. A 2.0 M potassium chloride solution is prepared by dissolving 37.3 g of potassium chloride in distilled water. What is the volume of the solution formed?(Relative atomic masses: K = 39.1, Cl = 35.5) b. What volume of 0.05 M Na2CO3 solution can be prepared from 2.65 g of Na2CO3?(relative atomic mass Na=23.0, C=12.0, O=16.0) c. Which of the following apparatus is usually used to deliver 25.0 cm3 of a solution into a conical flask?A. BuretteB. PipetteC. Beaker D. Volumetric flaskarrow_forward2arrow_forwardA chemical reaction doesn't necessarily involve electrons. °F Mostly sunny F1 O ! 1 Q A N True F2 2 W S F3 Alt -8+ X #M 3 E F4 D ta + $ 4 C F5 R 2 F % 5 F6 V T G F7 6 Y B F8 & 7 H U N F9 *00 8 J F10 ( 9 M O False K GO F11 O @ ) 0 < F12 L P Altarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY