
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
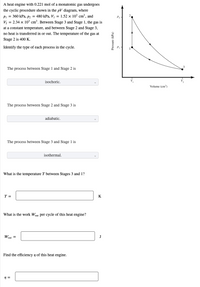
Transcribed Image Text:A heat engine with 0.221 mol of a monatomic gas undergoes
the cyclic procedure shown in the pV diagram, where
Pi = 360 kPa, p2 = 480 kPa, Vị = 1.52 × 10³ cm³, and
V, = 2.34 x 103 cm³. Between Stage 3 and Stage 1, the gas is
P2 -
at a constant temperature, and between Stage 2 and Stage 3,
no heat is transferred in or out. The temperature of the gas at
Stage 2 is 400 K.
Identify the type of each process in the cycle.
The process between Stage 1 and Stage 2 is
isochoric.
Volume (em)
The process between Stage 2 and Stage 3 is
adiabatic.
The process between Stage 3 and Stage 1 is
isothermal.
What is the temperature T between Stages 3 and 1?
Т-
K
What is the work Wout per cycle of this heat engine?
Wout =
Find the efficiency y of this heat engine.
Pressure (kPa)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Helium (He), a monatomic gas, fills a 0.036 3 m³ container. The pressure of the gas is 5.3 × 105 Pa. How long would a 0.25 hp engine have to run (1 hp = 746 W) to produce an amount of energy equal to the internal energy of this gas? t = iarrow_forwardThe heat engine shown in the figure uses 2.0 mol of a monatomic gas as the working substance. (Figure 1) igure p (kPa) 600- 400 200 0 0 0.025 0.050 V (m³) 1 of 1 Part E part. What is the engine's thermal efficiency? Express your answer using two significant figures. η = Submit VE ΑΣΦ Request Answer ? %arrow_forwardSuppose a monatomic ideal gas is changed from state A to state D by one of the processes shown on the PV diagram. PA Isotherms P₂2 atm P₁ atm A kJ E IN T B C 1 1 L I 4.00L 8.00L 16.0L V where P₁ = 1.10 and P₂ = 2.20. mat is the total work done on the gas if it follows the constant-temperature path AC followed by the constant-pressure path CD?arrow_forward
- The heat engine shown in the figure uses 2.0 mol of a monatomic gas as the working substance. (Figure 1) Figure p (kPa) 600 400 200 0 0 0.025 2 3 0.050 ▼ Determine AEth, Ws, and Q for 3→1. Enter your answers in joules and separated by commas. ► View Available Hint(s) AEth, Ws, Q = Submit Part E η = V What is the engine's thermal efficiency? Express your answer as a percentage. ► View Available Hint(s) VG ΑΣΦ Submit ΑΣΦ ? % ? Jarrow_forwardStill think that unit conversion isn't important? Here is a widely publicized, true story about how failing to convert units resulted in a huge loss. In 1998, the Mars Climate Orbiter probe crashed into the surface of Mars, instead of entering orbit. The resulting inguiry revealed that NASA navigators had been making minor course corrections in SI units, whereas the software written by the probe's makers implicitly used British units. In the United States, most scientists use Sl units, whereas most engineers use the British, or Imperial, system of units. (Interestingly, British units are not used in Britain.) For these two groups to be able to communicate to one another, unit conversions are necessary. The unit of force in the SI system is the newton (N), which is defined in terms of basic Sl units as 1 N=1 kg · m/s?. The unit of force in the British system is the pound (lb), which is defined in terms of the slug (British unit of mass), foot (ft), and second (s) as 1 lb =1 slug ·…arrow_forwardThe PV diagram shows the compression of 40.9 moles of an ideal monoatomic gas from state A to state B. Calculate Q, the heat added to the gas in the process A to B. Data: PA= 1.90E+5 N/m2 VA= 1.83E+0 m3 PB= 1.01E+5 N/m2 VB= 8.90E-1 m3›44arrow_forward
- A sample of n = 2.00 moles of monoatomic ideal gas expands adiabatically, the work done on the gas is W = -5.00 x 103 J. The initial temperature and pressure of the gas are Ti = 600 K and Pi = 4.05 x 105 Pa. Calculate: a) the final temperature of the gas; b) the final pressure of the gas. R = 8.314 J/mol Karrow_forwardA container having a volume of 2.30 L holds 1.80 g of helium gas at a temperature of 29.0 °C. (a) Find the pressure in the container. P = atm (b) Helium behaves as an ideal monoatomic gas. Find the internal energy of the system. Eint =arrow_forwardA heat engine with 0.221 mol of a monatomic gas undergoes the cyclic procedure shown in the ?? diagram, where ?1=360 kPa, ?2=480 kPa, ?1=1.52×103 cm3, and ?2=2.34×103 cm3. Between Stage 3 and Stage 1, the gas is at a constant temperature, and between Stage 2 and Stage 3, no heat is transferred in or out. The temperature of the gas at Stage 2 is 400 K. Identify the type of each process in the cycle. The process between Stage 1 and Stage 2 is The process between Stage 2 and Stage 3 is The process between Stage 3 and Stage 1 is What is the temperature ? between Stages 3 and 1? ?= What is the work ?out per cycle of this heat engine? ?out= Find the efficiency ? of this heat engine. ?=arrow_forward
- Suppose polarized light passes through a polarizing filter with its axis at an angle of 19° to the direction of polarization. To what percent of its original value is the intensity of the polarized light reduced?arrow_forwardAn ideal monatomic gas undergoes changes in pressure and volume, as shown in the pV diagram below. The initial volume is 0.02 m3 and the final volume is 0.10 m3. The initial pressure is 1 atm and the final pressure is 2 atm. Recall that 1 atm = 101.3 kPa. (a) Calculate the magnitude, or absolute value, of the work done on the gas in this process. Answer = - 13429J (c) The initial temperature of the gas is 308 K. Calculate the temperature of the gas at the end of the process. Answer = 3080 k Just need answer with the following: (d) What is the change in thermal energy for the gas in this process? (e) Calculate the quantity of heat transfer added to (positive) or removed from (negative) the gas during this process.arrow_forwardA particular thermodynamic cycle acting on a monatomic ideal gas (y = 1.67) includes an isobaric expansion, an isochoric cooling, and then a isothermic contraction. The PV diagram is shown in the image below. P V The isobaric expansion occurs at a pressure of 2.265 × 105 Pa and changes the volume of the gas from 5.9 × 10 2 m³ to 10.98 × 10-2 m³. What is the efficiency of the process?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON