
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Please help with these 2 HW Questions

Transcribed Image Text:Which is produced when magnesium metal reacts with a strong acid?
A) H2
B) MgH2
C) Mg ()
D) Mg-2H20
O A
O B
OD
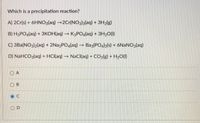
Transcribed Image Text:Which is a precipitation reaction?
A) 2Cr(s) + 6HNO3(aq) →2Cr(NO3)3(aq) + 3H2(g)
B) H3PO4(aq) + 3KOH(aq) K3PO4(aq) + 3H20(1)
C) 3Ba(NO3)2(aq) + 2Na3PO4(aq) –→ Baz(PO4)2(s) + 6NANO3(aq)
D) NaHCO3(aq) + HCI(aq) → NaCl(aq) + CO2lg) + H2O(1)
O A
O B
O D
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Try Again Row 3: Your answer is wrong. In addition to checking your math, check that you used the right data and DID NOT round any intermediate calculations. A student runs two experiments with a constant-volume "bomb" calorimeter containing 1100. g of water (see sketch at right). First, a 7.000 g tablet of benzoic acid (CH₂CO₂H) is put into the "bomb" and burned completely in an excess of oxygen. (Benzoic acid is known to have a heat of combustion of 26.454 kJ/g.) The temperature of the water is observed to rise from 15.00 °C to 53.26 °C over a time of 12.3 minutes. Next, 5.420 g of acetylene (C₂H₂) are put into the "bomb" and similarly completely burned in an excess of oxygen. This time the temperature of the water rises from 15.00 °C to 74.42 °C. Use this information, and any other information you need from the ALEKS Data resource, to answer the questions below about this reaction: Is this reaction exothermic, endothermic, or neither? If you said the reaction was exothermic or…arrow_forward3. A sample of glucose reacts in anaerobic respiration. The right-hand box below shows a particle diagram of the moles of substances present after the reaction is complete. On a piece of paper draw the "Before" box as shown and draw a particle diagram of the reactant molecules that produced the mixture shown on the right. Key Before = C₂H5OH = CO₂ = C6H12O6 After You will need to draw a diagram to answer this question. On a piece of paper, draw the "Before" box as shown, and then draw a particle diagram of the reactant molecules that produced the mixture shown on the right. Upload an image of your drawing by clicking "Upload files" or by dragging and dropping your file into the box. Or, use your device's camera to take a photo of your work by clicking the camera icon.arrow_forwardFill in the missing products or reactants for the following hydrogenation reactions.arrow_forward
- Under standard conditions, how much energy can be produced (i.e. what is ΔG'o ) when 1 mole of NADH passes its electrons to O2 to generate H2O? 1. -19 kJ 2 -30 kJ 3 -220 kJ 4 -1900 kJarrow_forwardX D2 Grades - CHE1011C41 Intro to CX signmentProblemID=195546712 100 11 Part A O exothermic reaction O endothermic reaction Classify reaction as exothermic or endothermic. Gas burning in a Bunsen burner: CH4(g)+20₂ (g)→CO₂ (g)+2H₂O(g) +802kJ Submit Part B Give AH for reaction above. Express your answer as an integer. Request Answer VE ΑΣΦ 1 Submit Part C Request Answer MasteringChemistry: Chapter 7 X + * lii ? kJ NO & Review Constants 43°F ^ @ (4¹) 2arrow_forwardQuestion is attached in image. Be sure to use correct significant figures.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY