
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
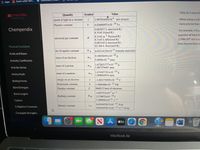
There are two pictures, one is the problem and the other is the values for physical constants.
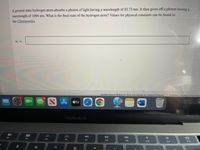
Transcribed Image Text:A ground state hydrogen atom absorbs a photon of light having a wavelength of 93.73 nm. It then gives off a photon having a
wavelength of 1094 nm. What is the final state of the hydrogen atom? Values for physical constants can be found in
the Chempendix.
Nf =
Question Source: McQuarrie, Rock, And Gallogly 4e- General Chemsitry
| Publisher: University Science Boo.
25
ムtv
W
MacBook Air
80
000
000
F3
DII
DD
F4
F5
F6
F7
F8
F9
F10
F11
F12
&
Transcribed Image Text:Cover Letter Sam...
Apps
FAQ: Do I need all
Value
almacmillan
learning
Quantity
Symbol
speed of light in a vacuum
2.99792458×10
8 m/s (exact)
When doing a calce
C
Planck's constant
6.62606957×10
-34
J•s
more precise than
Chempendix
0.082057 L·atm/(mol·K)
8.3145 J/(mol·K)
3 .
For example, if the
question all have th
8.3145 m Pa/(mol·K)
8.3145 L·kPa/(mol·K)
0.083145 L-bar/(mol·K)
62.364 L·Torr/(mol·K)
universal gas constant
R
2.998x108 m/s is su
limit the precision of
Physical Constants
the Avogadro constant
NA
6.02214129x10
23
formula units/mol
Acids and Bases
9.10938291x10 -28
mass of an electron
m e
-4
5.4858x10 amu
Activity Coefficients
-24
1.672621777x10
1.007276467 amu
mass of a proton
m p
Activity Series
-24
1.674927351x10
Amino Acids
mass of a neutron
1.008664916 amu
charge on an electron
-1.602176565×10 -19
e
Boiling Points
Boltzmann constant
k
1.3806488×10-23 J/K
Bond Energies
Faraday constant
F
96485 C/mol of electrons
1.097373×10 7 m-1
-18 j
Bond Lengths
Rydberg constant
RH
2.179872×10
Codons
3.289842x10
15
-1
S
8.854187817×10
-12
F/m
Electric constant
Colligative Constants
8.854187817x10-12 c2 /(J-m)
Conjugate Strengths
25
étv
W
MacBook Air
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- these are 2 different questions please label as 1 and 2arrow_forwardIn the laboratory a "coffee cup" calorimeter, or constant pressure calorimeter, is frequently used to determine the specific heat of a solid, or to measure the energy of a solution phase reaction. Water Thermometer Metal sample 2003 Thomson-Brooks/Cole Stirring rod Since the cup itself can absorb energy, a separate experiment is needed to determine the heat capacity of the calorimeter. This is known as calibrating the calorimeter and the value determined is called the calorimeter constant. One way to do this is to use a common metal of known heat capacity. In the laboratory a student heats 100.00 grams of nickel to 98.10 °C and then drops it into a cup containing 79.36 grams of water at 23.62 °C. She measures the final temperature to be 32.36 °C. Using the accepted value for the specific heat of nickel (See the References tool), calculate the calorimeter constant. Calorimeter Constant = J/°Carrow_forwardIn the laboratory a "coffee cup" calorimeter, or constant pressure calorimeter, is frequently used to determine the specific heat of a solid, or to measure the energy of a solution phase reaction. Thermometer Stirring rod Since the cup itself can absorb energy, a separate experiment is needed to determine the heat capacity of the calorimeter. This is known as calibrating the calorimeter and the value determined is called the calorimeter constant. One way to do this is to use a common metal of known heat capacity. In the laboratory a student heats 97.63 grams of nickel to 97.98 °C and then drops it into a cup containing 78.82 grams of water at 23.75 °C. She measures the final temperature to be 32.34 °C. Water- Metal sample Using the accepted value for the specific heat of nickel (See the References tool), calculate the calorimeter constant. Thomndrc Calorimeter Constant = | J/°C.arrow_forward
- Res Classify each function as a path function or a state function. Path function State function Answer Bank distance traveled enthalpy energy work heatarrow_forwardA 139-lb student races up stairs with a vertical height of 5.5 m in 5.5 s to get to a class on the second floor. How much power in watts does the student expend in doing work against gravity?arrow_forwardUse the following data to answer part A: mixing NaOH(s) into plain water released 1541 J of heat mixing NaOH(s) into HCl(aq) released 3498 J of heat mixing NaOH(aq) into HCl(aq) released 2000 J of heat Show that this data either supports or contradicts Hess’s Law and be clear in your explanation by using chemical equations and numerical calculations. Assume that all three parts use the same amount of NaOH and for the two parts using HCl(aq), the same amount of HCl was used as well. Hint: The sign of your values are important.arrow_forward
- Hi I am having trouble answering this question. Thank you.arrow_forwardIn the laboratory a "coffee cup" calorimeter, or constant pressure calorimeter, is frequently used to determine the specific heat of a solid, or to measure the energy of a solution phase reaction. Thermometer Metal sample ©2003 Thomson-Brooks/Co Stirring rod Since the cup itself can absorb energy, a separate experiment is needed to determine the heat capacity of the calorimeter. This is known as calibrating the calorimeter and the value determined is called the calorimeter constant. One way to do this is to use a common metal of known heat capacity. In the laboratory a student heats 93.37 grams of zinc to 98.36 °C and then drops it into a cup containing 81.25 grams of water at 21.46 °C. She measures the final temperature to be 28.84 °C. Using the accepted value for the specific heat of zinc (See the References tool), calculate the calorimeter constant. Calorimeter Constant = J/°Carrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY