
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
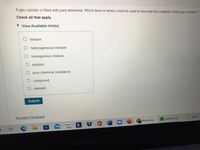
Transcribed Image Text:A gas cylinder is filled with pure ammonia. Which term or terms could be used to describe the contents of this gas cylinder?
Check all that apply.
View Available Hint(s)
mixture
heterogeneous mixture
homogenous mixture
O solution
pure chemical substance
O compound
O element
Submit
Provide Feedback
66°F
Mastering...
Spotify Free
B.
立
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Can someone provide a step-by-step answer for the following blanks in the second table? Thank you in advance!arrow_forwardP 5 2 ✓ C Home Insert Draw Design 2- m. AutoSave OFF Paste Page 1 of 1 Calibri (Bo... v 12 B I 0 words V 4 ab X₂ V Layout A^ A x² A V References Aa ✓ Dv 1 V English (United States) Mailings Review View B-E 1¹E == number of moles of water = V Tell me A↓ Document2 3 Aa BbCcDdEe Normal AaBbCcDdEe No Spacing 4 + Aa BbCcDc AaBb CcDd E€ Aa Bb Heading 1 Heading 2 Title 5 In Part B of the experiment, a student mixes 30.0 mL of 1.1 M HCl(aq) with 30.0 mL of 1.000 M NaOH(aq) in a well-insultated calorimeter and observes that the temperature of the solution increases by 5.75 °C. These are similar conditions as the previous two problems. How many moles of water are formed? Styles Pane 6 Focus Dictate E mol |||| I Share Comments Sensitivity Editor + 220%arrow_forward5. Generally, in order to do a stoichiometry problem, you need a complete balanced equation. However, in some cases it is possible to do some stoichiometric calculations without the complete equation as long as a particular element is present in only one reactant and one product. For example, if you were told that an experiment converted FeCl3 to Fe3O4 and that those were the only reactants and products that contained iron (Fe), you would know that there would have to be 3 moles of FeCl3 for every 1 mole of Fe;O4 or else the iron would not balance. You would not know for sure whether the balancing coefficients were actually 3 and 1 (They might be 6 and 2, 9 and 3, 12 and 4, etc) but you would know that they had to be in a 3 to 1 ratio and that would be enough to relate those two compounds with the correct stoichiometry. a) In Part I of your experiment, potassium carbonate (K,CO3) was the only reactant which contained carbon (C) and strontium carbonate (SrCO3) was the only product…arrow_forward
- A bedroom has a volume of 108 m^3. What is it volume in cubic kilometers (Km^3)?arrow_forwardDocume x → C Netflix Thermo X Cosmet x C Laptops x M Inbox (7 X learn.canterbury.ac.uk/ultra/courses/_19016_1/outline/edit/document/_3537106_1?courseld=_19016_1&view=content WOWER Saturati x C Buy TCL X a Amazon x C Show Y X G what's X Email - X យ ☆ b The dia x 90 h * ☐ 3. Calculate AG" and AG for the isomerization of dihydroxyacetone phosphate (DHAP) to glyceraldehyde 3-phosphate (GAP). This reaction takes place in glycolysis. At equilibrium, the ratio of GAP to DHAP is 0.0475 at 25°C (298 K) and pH 7. If the initial concentration of DHAP is 2 x 10 4M, and that of GAP is 2 x 10 M would the reaction be spontaneous under these conditions? (Answer: AG" = -RTINK= 7.54 kJ/mol, AG = -3.86 kJ/mol. Therefore, the reaction is spontaneous at the given concentrations) V :arrow_forwardCGE 32023#/ Dr. W. Scott Pers... Part C m = Submit Part D ΔΗ = How many grams of MgO are produced during an enthalpy change of -232 kJ ? Express your answer in grams to three significant figures. 0/ IVD| ΑΣΦ Submit Provide Feedback Krause etal_2007... Paraphrasing Paraphrasing Request Answer MacBook Air W aaac 2 0 0 ссссс VE ΑΣΦ Request Answer C 2. Ć Tool... Tool... ? How many kilojoules of heat are absorbed when 40.8 g of MgO(s) is decomposed into Mg(s) and O2 (g) at constant pressure? Lxpress your answer in kilojoules to three significant figures. 60 ? g X C C Ⓒ ✰ ✰ ☐ kJ Sentence Checker... | Inc. All rights reserved. | Terms of Use | Privacy Policy | Permissions Contact Us | + Update Next >arrow_forward
- write down the correct answer to a, b,c, darrow_forwardMr. Nugent (he/him|they/th... Diana Nguyen 2 jocelyn p Chem Classwork for Che X Balancing Eqnser Presentation Sess X Counting At 0ogle.com/presentation/d/1auph8DuajnWJDcUF7B02ieDMk6NBNvvXseY.. okmarks Links Attendance Drives A Classroom M Gmail Phys Morning Chem Morn ce the chemical equation: As + Na AsO, + _ H, NaOH →arrow_forwardme x C Thermo X Cosmet x C Laptops x M Inbox (7 X C Buy TCL X learn.canterbury.ac.uk/ultra/courses/_19016_1/outline/edit/document/_3537106_1?courseld=_19016_1&view=content T K Saturat X ZA a Amazon x C Show Ye X G what's a X Email- 1 X ☆ b The dia x h EX E ct the rato at which + 4. Consider the interconversion of A and B. Suppose in the absence of an enzyme, the forward rate constant KF is 104 s¹ and the reverse rate constant KR is 106 S¹. Calculate the equilibrium constant K. How would the presence of an enzyme affect the value of K? Karrow_forward
- I cannot understand why H2 is being a limiting reactant in this case.arrow_forwardCHRIRAD mework WP NWP Assessment Player Ul Appli X tion.wiley.com/was/ui/v2/assessment-player/index.html?launchld=d7fbf299-2b78-4aab-9fe4-25d65ce73435#/question/5 Question 6 of 6 W Carbon tetrachloride (CC14) was prepared by reacting 119 g of carbon disulfide and 119 g of chlorine. Calculate the percent yield if 65.0 g of CCl4 were obtained from the reaction CS₂+3 Cl₂ → CCl4 + S₂Cl2. Save for Later + % yield Want to see a relevant text example? GO Tutorial -/2 = O Search کا Attempts: 0 of 5 used Submit Answerarrow_forwardA openvellum.ecollege.com + P Course.. Self Assessment Exercise 3.1 - Enhanced - with Feedback 1 of 11 Part A Carbon monoxide and oxygen react to form carbon dioxide according to the following balanced chemical equation 2 CO(g) + O2(g) →2 CO2(g) If CO(g) and O2(g) are combined in the ratio shown in the diagram below, where the black spheres represent carbon atoms and the red spheres represent oxygen atoms, which molecules will remain when the reaction is complete? • View Available Hint(s) Only CO2 molecules O Co2 and O, molecules Co, and CO molecules CO and O, molecules Submit Pearsonarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY