
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
Don't copied from other sources plzz..
Explain all step don't skip any step
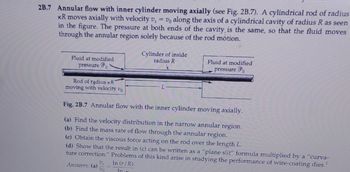
Transcribed Image Text:2B.7 Annular flow with inner cylinder moving axially (see Fig. 2B.7). A cylindrical rod of radius
KR moves axially with velocity v₂ = Vo along the axis of a cylindrical cavity of radius R as seen
in the figure. The pressure at both ends of the cavity is the same, so that the fluid moves
through the annular region solely because of the rod motion.
Fluid at modified
pressure
Rod of radius KR
moving with velocity vo
Cylinder of inside
radius R
Answers: (a) V₂
Vo
Fluid at modified
pressure Po
Fig. 2B.7 Annular flow with the inner cylinder moving axially.
(a) Find the velocity distribution in the narrow annular region.
(b) Find the mass rate of flow through the annular region.
(c) Obtain the viscous force acting on the rod over the length L.
(d) Show that the result in (c) can be written as a "plane slit" formula multiplied by a "curva-
ture correction." Problems of this kind arise in studying the performance of wire-coating dies.¹
In (r/R)
In
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Step 1: Stating the information from the diagram
VIEW Step 2: Finding the velocity distribution in the annular region by substituting Newton's law of viscosity
VIEW Step 3: By integral balance finding the mass flow rate through the annular region
VIEW Step 4: By substituting the velocity distribution equation finding the viscous force acting on the road.
VIEW Solution
VIEW Trending nowThis is a popular solution!
Step by stepSolved in 5 steps with 7 images

Knowledge Booster
Similar questions
- 4. Explain what happens during hot working. How is this different than cold working?arrow_forwardNote: The purge rate is the ratio between the molar flow rate of the purge stream and the molar flow rate of the feed.arrow_forwardFor a batch rectifier with appreciable column holdup, why do tray compositions change less rapidly than they do for a rectifier with negligible column holdup, and why is the separation improved?arrow_forward
- write a note on 2 Dimensional Defects(All of them), Electron Microscope(TEM, SEM, STM) support your writing with picturesarrow_forwardUse Graphical representation to show how crystal growth rate depends on crystallization temperature. Label Tm and Tg on the x axis and label the region where you will find large crystals to nucleate and growarrow_forwardDerivation of the general equation for interpolation: develop from scratch the equation interpolation you need to developarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The