
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Question

Transcribed Image Text:The system below uses two heat engines, where the waste heat from HE1 is used as the heat source for HE2.
a) Find the overall efficiency of the system shown below as a function of the two individual efficiencies. In other words, find \( \eta_{\text{overall}} = f(\eta_1, \eta_2) \).
b) Based on part a, can the efficiency of the two heat engines ever be greater than 100%? If multiple (infinite?) heat engines are chained together in the same way, so that each heat engine is powered by the waste heat of the one before it, can the overall efficiency ever be greater than 100%?
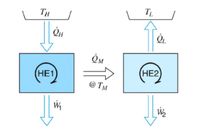
Transcribed Image Text:The diagram illustrates a two-stage heat engine system, consisting of two heat engines, labelled HE1 and HE2.
- **HE1** operates between a high temperature reservoir (denoted as \( T_H \)) and is supplied with heat energy \( \dot{Q}_H \). It generates work output \( \dot{W}_1 \).
- **HE2** operates between \( HE1 \) and a low temperature reservoir (denoted as \( T_L \)). \( HE2 \) receives the transferred heat energy \( \dot{Q}_M \) at an intermediate temperature \( T_M \) and transfers heat \( \dot{Q}_L \) to \( T_L \) while producing work \( \dot{W}_2 \).
Arrows represent the direction of heat and work flows. The cyclic arrows in HE1 and HE2 depict the cyclical nature of the engines' operation.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 19 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- I have been working on this problem for a while and keep getting wrong answers with many different meathods how do I solve for Q0 here?arrow_forwardThermodynamics question 3 in need the answer pleasearrow_forward1.2 A reversible heat pump is used to maintain a temperature of 0°C in a refrigerator when it rejects the heat to the surrounding at 25°C. (i) If the heat removal rate from the refrigerator is 1440 kJ/min, determine the COP of the machine and work input required. (ii) If the required input to run the pump is developed by a reversible engine which received heat at 380°C and rejects heat to atmosphere, then determine the overall COP of the system.arrow_forward
- Thermo review I cannot solve the last part of this question- thank you! A heat engine with a thermal efficiency of 45% rejects 1000 kJ/kg of heat to a low temperature sink. d) A Carnot heat pump operates between 273 K and 313 K, determine its Coefficient of Performance.arrow_forwardWhich of the following are correct and valid for the Kelvin-Plank statement? O a. Any device O b. Kelvin- Planck O c. It is possible for any O d. No heat engine can have a thermal O e. Kelvin- Planck O f. It is impossible O g. It is impossible for any device O h. It is impossible that statement statement to construct to construct of the second law is device that a device that a device that violates of the efficiency of 100 second law is the that operates in a cycle and produces no effect other than Kelvin- operates in a cycle and produces no effect other than operates negative statement, and a operates on a cycle to receive heat from a single reservoir Planck percent, or as for a positive statement, and a positive on a cycle to receive heat statement power plant to operate, the working fluid also violates negative statement the from a statement the transfer the transfer single reservoir and Clausius cannot be can be of heat from and of heat from a lower- temperature body to a higher-…arrow_forwardReferring to the reversible heat pump cycle shown in the figure, p1 = 14.7 Ibf/in?, p4 = 27.8 lbf/in?, v1 = 12.6 ft³/Ib, v4 = 8.0 ft³/lb, and the gas is air obeying the ideal gas model. P4 pi TH V4 VI Determine TH, in °R, and the coefficient of performance. Step 1 Determine TH, in °R. TH = °R Save for Later Attempts: 0 of 1 used Submit Answer Step 2 The parts of this question must be completed in order. This part will be available when you complete the part above.arrow_forward
- 2. A heat pump is like a refrigerator in that it uses work to take heat from a cold source (Tc, the inside of a house) and dumps it into a hot source (Th, usually the outdoor environment). a) Use the second law to find the magnitude of the work |w| needed to extract |qc] from the cold source and dump into the hot source. b) Assume all processes are reversible and the second law to explain why |w| > 0 if lgc] > 0 in part a).arrow_forwardThermodynamics- hand write plsarrow_forwardQuestion 5 A cyclic system exchanges heat with 3 reservoirs. The system receives 990 J from a reservoir at 1,236 Kand 542 J from a reservoir at 766 K. It rejects an unknown quantity of heat to a reservoir at 256 K. What is the maximum possible efficiency of the system? Give your answer as a percentage to 1 decimal place (e.g. 12.3% would be input as 12.3).arrow_forward
- 3.5 A novel heat engine is being considered for producing work from the thermal energy contained in the wastewater discharge from a large food-processing plant. On an average day, 35 ° C wastewater leaves the plant and is discharged into the ocean at 1,000 gallons per minute. If the average annual ocean temperature is 10 ° C at that location, what is the maximum power that could be produced by this heat engine? What would be the thermal cycle efficiency of your maximum power engine? You can assume that the heat capacity of water at constant pressure is constant at 4,200 J/kg K.arrow_forwardPlease show steps I keep coming up with -0.0044arrow_forwardb) Steam generators are a type of heat exchanges that are used in power plants to generate steam at desired pressure and temperature (Fig. Q1.b). In a steam generator, saturated liquid water at 30°C enters a 60-mm diameter tube at the volume flow rate of 12 L/s. After exchanging heat with hot gas, the water changes to steam and leaves the generator at a pressure of 9 MPa and a temperature of 400°C. During this process, the diameter of the water/steam tube does not change. Steam out Gas out Hot gas in Liquid water in Fig 2. Schematic of the steam generator in Q1.b. (i) Calculate the steam mass flow rate (ii) What is the inlet velocity of the steam? (iii) What is the exit velocity of the steam? (iv) Calculate the rate of heat transfer (in MW) required to change the phase of liquid water to steam.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY