
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
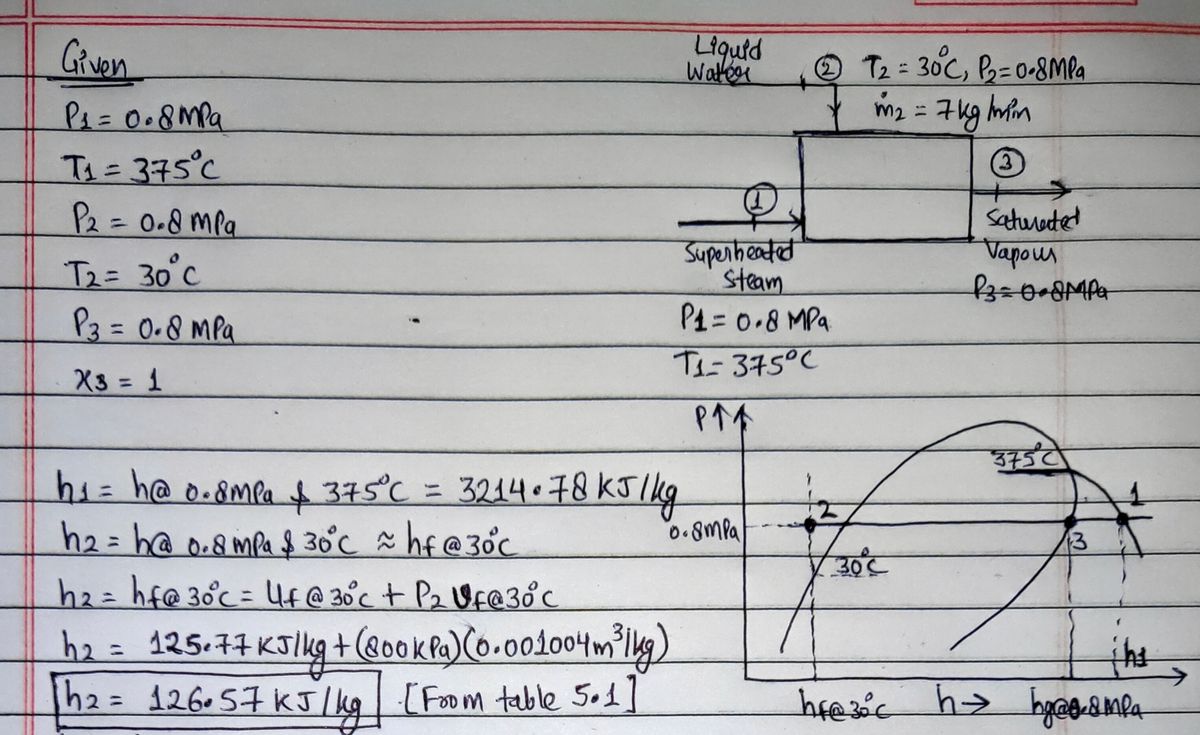
a desuperheater insulated unit (figure F) converts the inputs into a
saturated vapor stream. Determine the mass flow rate of the superheated
stream.
. Determine the enthalpy of liquid water both from table 5.1, table 5.4.
What is your conclusion from this comparison?

Transcribed Image Text:Internal Energy, kJ/kg
Specific Volume, m³/kg
Sat. Liquid
UL
Evap.
ULV
Sat. Vapor
Sat. Vapor
Temp.
(°C)
Sat. Liquid
vL
Evap.
VLV
Press.
UV
(kPa)
0.01
0.6113
0.001000
206.131
206.132
2375.33
2375.33
2361.27
147.118
106.377
77.925
20.97
2382.24
2389.15
5
0.8721
0.001000
147.117
41.99
62.98
2347.16
10
1.2276
0.001000
106.376
2333.06
2396.04
2402.91
15
1.705
0.001001
77.924
20
2.339
0.001002
57.7887
57.7897
83.94
2318.98
2304.90
43.3593
32.8932
104.86
2409.76
2416.58
25
3.169
0.001003
43.3583
30
4.246
0.001004
32.8922
125.77
2290.81
35
5.628
0.001006
25.2148
25.2158
146.65
2276.71
2423.36
167.53
2262,57
2430.11
2436.81
19.5229
0.001008
0.001010
40
7.384
19.5219
45
9.593
15.2571
15.2581
188.41
2248.40
12.0318
209.30
2234.17
2443.47
2450.08
2456.63
50
12.350
0.001012
12.0308
LIQUID WATER T, = 30 °C ,e = 0. 8 MPea
niz= 7 kg/min
2219.89
9.56835
7.67071
55
15.758
0.001015
9.56734
230.19
251.09
2205.54
0.001017
0.001020
60
19.941
7.66969
65
25.03
6.19554
6.19656
272.00
2191.12
2463.12
70
31.19
0.001023
5.04114
5.04217
292.93
2176.62
2469,55
75
38.58
0.001026
4.13021
4.13123
313.87
2162.03
2475.91
80
47,39
0.001029
3.40612
3.40715
334.84
2147.36
2482.19
85
57.83
0.001032
2.82654
2.82757
355.82
2132.58
2488.40
90
70.14
0.001036
2.35953
2.36056
376.82
2117.70
2494.52
SATURAT ED
95
84.55
0.001040
1.98082
1.98186
397.86
2102.70
2500.56
VAPOR
100
101.3
0.001044
1.67185
1.67290
418.91
2087.58
2506.50
SUPERHEATED
STE AM
105
= 0.8 MPa
120.8
0.001047
1.41831
1.41936
440.00
2072.34
2512.34
110
143.3
0.001052
1,20909
1.03552
1.21014
461.12
2056.96
2518.09
0.001056
Pz = a.8 MPa
115
169.1
1.03658
482.28
2041.44
2523.72
120
198.5
0.001060
0.89080
0.89186
503.48
2025.76
2529.24
125
232.1
0.001065
0.76953
0.77059
2009.91
2534.63
Tz= 375°C
524.72
130
270.1
0.001070
0.66744
0.66850
546.00
1993.90
2539.90
135
313.0
0.001075
0.58110
0.58217
567.34
1977.69
2545.03
140
361.3
0.001080
0.50777
0.50885
588.72
1961.30
2550.02
145
415.4
0.001085
0.44524
0.44632
610.16
1944.69
2554.86
2559.54
2564.04
150
475.9
0.001090
0.39169
0.39278
631.66
1927.87
155
543.1
0.001096
0.34566
0.34676
653.23
1910.82
2568.37
2572.51
2576.46
160
617.8
0.001102
1893.52
1875.97
0.30596
0.30706
674.85
165
700.5
0.001108
0.27158
0.27269
696.55
170
791.7
0.001114
0.24171
1858.14
1840.03
0.24283
718.31
2580.19
2583.70
175
892.0
0.001121
0.21568
0.21680
740.16
180
1002.2
0.001127
0.19292
0.19405
762.08
1821.62
185
1122.7
0.001134
0.17295
2586.98
0.17409
784.08
1802.90
190
0.001141
2590.01
1254.4
0.15539
0.15654
806.17
1783.84

Transcribed Image Text:tearn
cle
口
500 | Thermodynamics: Fundamentals and Applications for Chemical Engineers
TABLE 5.4-Properties of Compressed Liquid Water
H.
V
V
Temp.
V
H
(kJ/kg)
(kJ/kg)
(kJ/kg-K)
Tеmp.
(m³/kg)
U
(m³/kg)
(kJ/kg)
(kJ/kg)
(m'/kg)
(kJ/kg)
(kJ/kg)
(kl/kg-K)
(°C)
(m³/kg)
(kJ/kg)
(kJ/kg)
(kJ/kg-K)
(k/kg-K)
("C)
2000 kPa (212.42°C)
15000 kPa (342.24°C)
20000 kPa (365.81°C)
500kPa (151.86°C)
640.21
0.001177
906.42
0.001658
1585.58
1610.45
3.6847
0.002035
1785.47
1826.18
908.77
4.0137
1.8606
0.0000
Sat.
0.001093
639.66
Sat.
0.15
15.04
0.0004
0.000999
0.03
2.4473
0.000993
0.000990
0.20
20.00
0.0004
0.01
0.000999
0.01
0.51
2.03
0.001001
83.82
0.0001
0.000995
83.05
97.97
0.2934
0.000993
82.75
102.61
0.2922
20
0.001002
83.91
84.41
0.2965
85.82
20
0.001007
167.29
169.30
2962
0.001001
165.73
180.75
0.5665
0.000999
165.15
185.14
40
167.47
167.98
0.5722
40
0.5646
0.001008
0.001017
0.001016
250.73
252.77
.5716
0.001011
248.49
263.65
0.8231
0.001008
247.66
267.82
0.8205
60
251.00
251.51
0.8308
60
331.46
346.79
.8300
1.0739
335.24
1.0749
0.001028
334.38
336.44
80
0.001022
1.0655
0.001020
330.38
350.78
1.0623
80
0.001029
334.73
0.001043
418.36
420.45
0.001036
414.72
430.26
1.2954
0.001034
413.37
434.04
1.2917
100
0.001043
418.80
419.32
1.3065
100
1.5273
0.001059
502.84
504.96
1.3053
0.001052
498.39
514.17
1.5144
0.001050
496.75
517.74
1.5101
120
0.001060
503.37
503.90
120
589.20
1.7389
0.001079
588.02
590.18
1.5259
0.001071
582.64
598.70
1.7241
0.001068
580.67
602.03
1.7192
140
0.001080
588.66
140
160
0.001101
674.14
676.34
1.7373
0.001092
667.69
684.07
1.9259
0.001089
665.34
687.11
1.9203
160
0.001127
761.46
1.9410
180
0.001116
753.74
770.48
2.1209
0.001112
750.94
773.18
2.1146
180
763.71
2.1382
2.3301
200
0.001156
850.30
852.61
200
0.001143
841.04
858.18
2.3103
0.001139
837.70
860.47
2.3031
220
0.001175
929.89
947.52
2.4952
0.001169
925.89
949.27
2.4869
10000 kPa (311.06°C)
1020.82
1038.99
2.6770
0.001205
1015.94
1040.04
2.6673
5000 kPa (263.99°C)
240
0.001211
0.001452
1393.00
0.001255
1114.59
1133.41
2.8575
0.001246
1108.53
1133.45
2.8459
Sat
0.001286
1147.78
1154.21
2.9201
1407.53
260
3.3595
280
0.001308
1212.47
1232.09
3.0392
0.001297
1204.69
1230.62
3.0248
0.000998
0.03
5.02
0.0001
0.000995
0.10
10.05
0.0003
300
0.001377
1316.58
1337.23
3.2259
0.001360
1306.10
1333.29
3.2071
20
0.001000
83.64
88.64
0.2955
0.000997
83.35
93.32
3.3978
0.2945
320
0.001472
1431.05
1453.13
3.4246
0.001444
1415.66
1444.53
40
0.001006
166.93
171.95
0.5705
0.001003
166.33
176.36
3.6074
3.8770
0.5685
340
0.001631
1567.42
1591.88
3.6545
0.001568
1539.64
1571.01
60
0.001015
250.21
255.28
0.8284
0.001013
249.34
259.47
0.8258
1702.78
1739.23
0.001823
80
0.001027
333.69
338.83
1.0719
0.001025
332.56
342.81
360
1.0687
100
0.001041
417.50
422.71
1.3030
0.001039
416.09
426.48
1.2992
50000 kPa
30000 kPa
120
0.001058
501.79
507.07
1.5232
0.001055
500.07
510.61
1.5188
0.0001
0.000977
0.20
49.03
-0.0014
0.25
29.82
0.000986
0.000989
140
0.001077
586.74
592.13
1.7342
0.001074
584.67
595.40
1.7291
0.2898
0.000980
80.98
130.00
0.2847
20
82.16
111.82
160
0.001099
672.61
678.10
1.9374
0.001195
670.11
681.07
1.9316
0.5606
0.000987
161.84
211.20
0.5526
40
0.000995
164.01
193.87
180
0.001124
759.62
765.24
292.77
0.8051
0.000996
0.001007
2.1341
0.001120
756.63
767.83
2.1274
0.8153
242.96
60
0.001004
246.03
276.16
200
0.001153
848.08
853.85
2.3254
0.001148
844.49
855.97
1.0561
324.32
374.68
1.0439
2.3178
80
0.001016
328.28
358.75
405.86
456.87
1.2703
0.001020
0.001035
0.001052
220
0.001187
938.43
944.36
2.5128
0.001181
934.07
945.88
2.5038
441.63
1.2844
100
0.001029
410.76
539.37
1.4857
240
0.001226
1031.34
1037.47
2.6978
0.001219
1025.94
524.91
1.5017
487.63
1038.13
2.6872
120
0.001044
493.58
622.33
1.6915
260
0.001275
1127.92
1134.30
2.8829
0.001265
608.73
1.7097
569.76
1121.03
1133.68
576.86
2.8698
3.0547
140
0.001062
0.001070
652.39
705.91
1.8890
280
0.001322
1220.90
1234.11
660.81
693.27
1.9095
160
0.001082
0.001091
735.68
790.24
2.0793
300
745.57
778.71
2.1024
0.001397
1328.34
1342.31
3.2468
180
0.001,105
819.73
875.46
2.2634
865.24
2.2892
0.001115
200
0.001130
831.34
961.71
2.4419
0.001141
0.001170
904.67
918.32
953.09
2.4710
220
0.001159
990.69
1049.20
2.6158
1042.60
2.6489
240
0.001192
1006.84
1138.23
2.7860
2.8242
0.001203
1078.06
1097.38
1134.29
260
0.001230
0.001242
1167.19
1229.26
2.9536
1190.69
1228.96
2.9985
280
0.001275
1258.66
1322.95
3.1200
1327.80
3.1740
0.001286
1287.89
300
0.001330
1353.23
1420.17
3.2867
1432.63
3.3538
0.001339
320
0.001400
1390.64
1522.07
3.4556
0.001403
0.001484
3.5425
1451.91
1501.71
1546.47
0.001492
3.6290
3.8100
340
3.7492
1555.97
1630.16
1626.57
1675.36
360
0.001627
1667.13
1746.54
1781.35
1837.43
4.0010
0.001588
380
0.001869
DTO D
Expert Solution
arrow_forward
Step 1
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Thermodynamic question How to draw the T-s diagram for the his problem? A volume flow of air at 500K, 20bar passes in a long pipe through a temperature reservoir that is kept at a temperature of 1000°C. The air exits at 1000K and 1.8 MPa. Consider air as ideal gas with variable specific heats, ? = 0.287 kJ/kg-Karrow_forwardIn the condenser of the power plant, Ammonia is isobarically condensed to saturated liquid state with 615.2kPa and x = 0.9, the mass flow rate is 4 t/h. The heat rejected from the condensing ammonia is added to heat water, which is heated from 277K to 281K. What is the mass flow rate of water in t/h. Also sketch the T-s and P-v diagrams.arrow_forwardEvaporator single effect is used to upgrade the concentrate 10000 kg/h of tomato juice than 5% solids the total to 30% solids total. Juice enters the Evaporator at 15 °C. The Evaporator is operated with Steam (quality 80%) in 143.27 kPa. The condition of vacuum in the Evaporator allow the juice to a boil on the 75 °C. The hot kind of material dilute is 4.1 kJ/(kg °C) and the product the concentrate is a 3.1 kJ/(kg °C). Count a. The Rate of the needs of steam = ...kg/h. b. The economic steam if the temperature is condensate is released at 75 °C. = ...(kg water evaporated/kg of steam)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY