Question
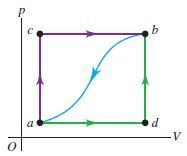
When a system is taken
from state a to state b in Fig.
along path acb, 90.0 J of heat flows
into the system and 60.0 J of work is
done by the system. (a) How much
heat flows into the system along path
adb if the work done by the system
is 15.0 J? (b) When the system is returned
from b to a along the curved
path, the absolute value of the work
done by the system is 35.0 J. Does
the system absorb or liberate heat? How much heat? (c) If Ua = 0 and
Ud = 8.0 J, find the heat absorbed in the processes ad and db.
Transcribed Image Text:a
d
V
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- What is the final equilibrium temperaturewhen 5 g of milk at 18◦C is added to 163 g ofcoffee at 90◦C? Assume the specific heats ofmilk and coffee are the same as that of water,and neglect the specific heat of the container.Answer in units of ◦Carrow_forwardThe pcessure of Imole of an ideal gas जरमाण ग cceasing at a rate of O.06K Pa/s and the temperature s increa Use the equation PV=8.317 to find the rate of %3D change uf tre volume when the prssure is 21xPa andthe temperature is 323 K. (Roundyour answer to tuo decimal places) 4.arrow_forwardTwo heat reservoirs (500K and 300K) operate a Carnot engine betweenthem. (a) If the engine receives 1000 J from the 500 K reservoir, what heatis rejected to the 300 K reservoir? (b) If the engine were operated in reverseas a refrigerator and received 1000 J from the 300 K reservoir, what heat isdelivered to the 500 K reservoir. (c) What is the heat equivalent to themechanical work required to operate the refrigerator in item b.arrow_forward
- A hot-air balloon is inflated by heating air at constant pressure. Assume the number of air moleculesinside the balloon remains constant and that it expands at atmospheric pressure. (a) How much work does the balloon do to expandagainst atmospheric pressure?arrow_forward(a) What is the efficiency (in percent) of a cyclical heat engine in which 45.0 kJ of heat transfer occurs to the environment for every 65.0 kJ of heat transfer into the engine? % (b) How much work (in k) does it produce for 950 kJ of heat transfer into the engine? kJ Additional Materials CSScanned with CamScanner TPeadinaarrow_forward
arrow_back_ios
arrow_forward_ios