Question
A container of neon gas has the following properties:
(1) 2.4 moles
(2) T = 400 K
(3) p = 0.8 atm.
Calculate the entropy in J K-1 as a number in normal form accurate to 1 decimal place (X.X or XX.X, etc.). Do not include units in your answer.
USE THE EXACT METHOD IN THE PICTURE BELOW, JUST SUB IN THE NEW VALUES IN THE QURSTION AND REPLACE THEM IN THE IMAGE PROVIDED.
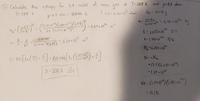
Transcribed Image Text:2 Calculate the entropy
for 2,3 moles of
at T: 289 K and p:0.8 atm
neon gas
T= 289 K
1 mol = 6,02<10 atons
Ne = 20.18 g
p=0.8 atm =
81040 Pa
m= 20.189
6.022치03
= 2.64 x 10%
3.35 x 10 ka
-23
2r (1.055 a 10 3)=
-34
*- 1.,055x 10 J-S
21040
(1:38 -10*)(289) = 2.03 × 103
k= 1.38|x 1o /K
-23
KT
NA 6.022 10
S= Nk [ln () + = (19.196) l
2.64 x10%
2.03 x 109
N=nNA
= (2.3) (6.022 지6)
*1.39 x 10*
S: 539.2
mol-k
NK : (1.3f- 10")(1381 10)
= 19.196
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Similar questions
- 1. The system below shows a box with two indistinguishable balls (filled in circles) and four possible positions, with only one ball allowed in each position. Assume that the system starts with a barrier between side 1 and side 2. Calculate the change in entropy if the barrier is removed and the balls are able to freely move to other positions. Give your answer in units of J/K. +arrow_forwardNEED ASAParrow_forwardi need the answer quicklyarrow_forward
- Please help mearrow_forwardAnswer in 90 minutes please.arrow_forwardDetermine which of the following are true or false and explain your answer: a) During an isothermal process, the entropy of an ideal gas stays constant if there is a net change in either pressure or volume b) Entropy generation takes place when there are irreversibilities in a cycle. c) The lower the isentropic efficiency of a turbine, the more power it will generate with the same input stream and same output pressure.arrow_forward
arrow_back_ios
arrow_forward_ios