
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
![\/
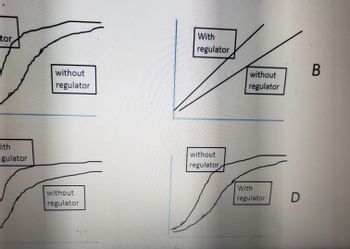
A compound was suspected of being a negative allosteric regulator of enzyme A. Which of the
following would be the expected Rate vs [S] in the absence and presence of the allosteric effector?
For each of these figures rate is graphed on the y-axis and substrate concentration is graphed on
the x-axis. (one best answer)
OA
OB
D](https://content.bartleby.com/qna-images/question/07b00a25-4bbc-4603-8563-821c16d74641/a240b66f-86a9-46ff-b05a-534e29366ea2/too5px_thumbnail.jpeg)
Transcribed Image Text:\/
A compound was suspected of being a negative allosteric regulator of enzyme A. Which of the
following would be the expected Rate vs [S] in the absence and presence of the allosteric effector?
For each of these figures rate is graphed on the y-axis and substrate concentration is graphed on
the x-axis. (one best answer)
OA
OB
D
Transcribed Image Text:tor
ith
egulator
without
regulator
6
without
regulator
With
regulator
without
regulator
without
regulator
With
regulator
D
B
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Solve the Michaelis-Menten equation for KM when vo=Vmax/2. What does this tell you about the relationship between substrate concentration and enzymes with high KM values? With low KM values?arrow_forward(i) Which graph indicates an enzymatic reaction without inhibitor?(ii) Which type of inhibitor is it? Briefly explain.(iii) Which graph indicates the highest concentration of inhibitor?(iv) Calculate the Vmax and Km of the graph showing an enzymatic reaction with the lowest concentration of inhibitor. Show the steps of calculation and unit in your answers. Keep 2 decimal places in your answers.arrow_forwardVo 60 50 40 30 20 mm/sec 0100 0 O 0 0 50 100 150 200 250 300 Substrate (mM) Use the Michaelis-Menten plot to answer this question. What is the estimated value of Vmax of the enzyme catalyzed reaction (square data points)? (choose the one best answer) 10arrow_forward
- Which of these statements about enzyme-catalyzed reactions is false? The activation energy for the catalyzed reaction is the same as for the uncatalyzed reaction, but the equilibrium constant is more favorable in the enzyme-catalyzed reaction. The Michaelis-Menten constant Km equals the [S] at which V = 1/2 V, max: At saturating levels of substrate, the rate of an enzyme-catalyzed reaction is proportional to the enzyme concentration. The rate of a reaction decreases steadily with time as substrate is depleted. If enough substrate is added, the normal V, of max a reaction can be attained even in the presence of a competitive inhibitor.arrow_forwardYou begin to study enzyme Z, which catalyzes a simple reversible reaction that interconverts compound S and compound P. You observe that the ∆G´° for the S to P conversion to be –6 kJ/mol, and that compound S has ∆G´° for binding to enzyme Z of –15 kJ/mol, while compound P has a ∆G´° for binding to enzyme Z of –13 kJ/mol. Please explain the effect of enzyme Z on conversion of S to P. (Your answer should include a graph qualitatively showing energy versus reaction progress; however, you still need to explain youranswer in words!) not sure how to make the correct graph.arrow_forwardThe initial velocities of two different enzyme-catalyzed reactions were measured over a series of substrate concentrations. The following results were obtained: Enyme A: KM = 1.5 mM, Vmax = 10 μM s-1 Enyme B: KM = 5.0 mM, Vmax = 85 µM s-1 (a) Which enzyme binds to its substrate more tightly (assume k.1 >> k₂ in the Michaelis-Menten model)? (b) Calculate the initial velocities of each reaction when the substrate concentration is 2.5 mM. (c) Calculate the Kcat of each enzyme if the total enzyme concentration is 100 nM. (d) Which enzyme is the more efficient catalyst? Explain your answer. The enzyme carbonic anhydrase is strongly inhibited by the drug acetazolamide. A plot of the initial reaction velocity (as a percentage of Vmax) in the absence and presence of the inhibitor is shown below. What type of inhibition is taking place? Explain your reasoning. V (% of Vmax) 100 50 0.2 0.4 No inhibitor Acetazolamide [S] (MM) 0.6 0.8 1arrow_forward
- For an enzyme-catalyzed reaction, the velocity was determined at two different concentrations of the substrate. Estimate the value of Vmax. [S] (MM) 10 20 88 nmol/min 79 nmol/min 67 nmol/min V(nmol/min) 51 nmol/min 27 Not enough information is given to form a reasonable estimation. 48arrow_forwardSelect the graph that correctly illustrates the effect of a positive modifier (effector) on the velocity curve of an allosteric enzyme. Place the correct graph in the set of axes. The solid blue curve represents the unmodified enzyme. The dashed green curve represents the enzyme in the presence of the effector. R is the highly active form of the enzyme and T is the less active form of the enzyme. Assume that this is a positively cooperative enzyme, meaning that the affinity for substrate increases with increasing substrate concentration.arrow_forwardGiven: Factor VIIa is a man-made protein analog to Factor VII, which is involved in coagulation. (Answer a, b, and c)a. Identify both a competitive and non-competitive inhibitor for Factor VIIa b. Draw a graph showing how both of these molecules will change the Velocity/[S] graph, and explain why this is the case. c. What would a graph look like if both inhibitors were added?arrow_forward
- In a uni uni enzyme reaction, what is the substrate concentration relative to Km when anenzyme operates at 0.95 * V. What about 0.99 * V?arrow_forwardThe Michaelis-Menten equation is often used to describe the kinetic characteristics of an enzyme-catalyzed reaction. Vmax [S] Km + [S] where v is the velocity, or rate, Vmax is the maximum velocity. K is the Michaelis-Menten constant, and [S] is the substrate concentration. A graph of the Michaelis-Menten equation is a plot of a reaction's initial velocity (ro) at different substrate concentrations ([S]). First, move the line labeled Vmax to a position that represents the maximum velocity of the enzyme. Next, move the line labeled 1/2 Vmax to its correct position. Then, move the line labeled Km to its correct position. Estimate the values for Vmax and Km- Vmax= µM/min v (µM/min) 300 275 250 225 200 175 150 125 100 75 50 Km = 25 K 0 10 20 30 V max 40 50 [S] (M) 1/2 V max Michaelis-Menten curve 60 70 80 90 100 HMarrow_forward5.50 1/V, min/umol 5.00 4.50 4.00 y = 0.9474x + 2.6649 y = 0.9997x + 2.032 0.00 1.00 2.00 2.50 3.00 1/[S], uM -1 Looking at the double reciprocal plot for an enzyme in the absence of inhibitor and in the presence of two concentrations of inhibitor, what would be the Vmax for the uninhibited enzyme? (bottom graph) Equation is given. Choose the one best answer. 3.50 3.00 2.50 2.00arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON