
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
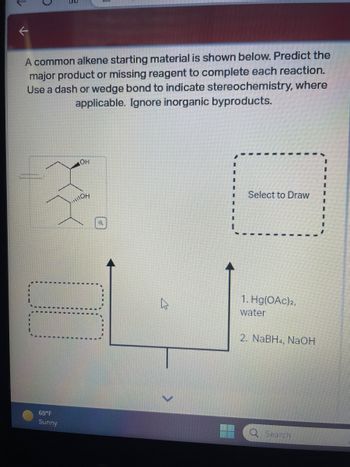
Transcribed Image Text:**Title: Predicting Major Products in Alkene Reactions**
**Introduction:**
The following exercise involves predicting the major product or identifying the missing reagent in a series of reactions starting with a common alkene. Use the concepts of stereochemistry to accurately depict the molecular structure, utilizing dash or wedge bonds as needed. Note that inorganic byproducts should be disregarded in your analysis.
**Starting Material:**
An alkene with two hydroxyl groups is depicted, showing the need for stereochemistry consideration (a wedge bond denotes one of the hydroxyl groups).
**Reactions:**
1. **Reagents:**
- First step: Hg(OAc)₂, water
- Second step: NaBH₄, NaOH
**Instructions:**
Analyze the reaction mechanism, considering the stereochemical implications of each step. Use the provided reagents to predict the outcome, focusing on the formation of the major product.
**Diagram Explanation:**
- The starting material is placed at the bottom.
- The reaction process is depicted by upward-pointing arrows leading to a placeholder for the resulting product.
- The final product box is labeled "Select to Draw," prompting engagement with the analysis.
**Conclusion:**
This interactive analysis helps reinforce understanding of reaction mechanisms and stereochemistry in predicting organic synthesis outcomes.
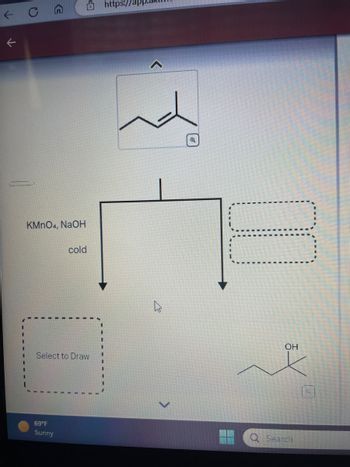
Transcribed Image Text:This image presents a reaction scheme featuring an alkene, which is shown at the top of the flowchart. The displayed chemical structure is a hydrocarbon chain with a double bond. Beneath this structure, a reaction condition is specified: potassium permanganate (KMnO₄) with sodium hydroxide (NaOH) in a cold environment. This indicates a syn-dihydroxylation reaction.
The flowchart divides into two paths:
1. **Left Path:**
- Contains a dashed box labeled "Select to Draw," suggesting an area where users can input or predict the resulting chemical structure of the reaction.
2. **Right Path:**
- Leads to another dashed box, implying another potential outcome or reaction step, but it is left blank.
At the bottom right, the resulting product of the reaction under these conditions is illustrated. The structure shows the alkene transformed into a diol, with added hydroxyl (OH) groups marking the syn addition.
**Additional Details:**
- A temperature indicator at the bottom reads "69°F Sunny," which is likely not related to the chemical content.
- The interface is part of an educational platform, indicated by the URL placeholder at the top of the image.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 6 steps with 6 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- curved arrows are used to illustrate the flow of electrons. using the provided starting structure, draw the curved electron- pushing arrows for the following reaction or mechanistic steps. be sure to account for all bond-breaking and bond-making steps. then draw any missing organic intermediates or products for this reaction. include all lone pairs in the structures. ignore inorganic byproducts, counterions, and solvents.arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting structure, draw the curved electron- pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. Then draw the organic product of this reaction. Include all lone pairs in the structures. Ignore inorganic byproducts and counterions. :0: I I H I I - + O Select to Add Arrows H2O, HCI H :CI:O I Iarrow_forwardDraw the major organic product(s) of the following reactions including stereochemistry when it is appropriate. H20/ H,SO, / HgSO4 CH,CH2-CEC-CH, • Use the wedge/hash bond tools to indicate stereochemistry where it exists. • If no reaction occurs, draw the organic starting material. Separate multiple products using the + sign from the drop-down menu.arrow_forward
- See image belowarrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting structure, draw the curved electron-pushing arrows for the following reaction or mechanistic steps. Be sure to account for all bond-breaking and bond-making steps. Then draw the organic product of this reaction. Include all lone pairs in the structures. Ignore inorganic byproducts, counterions, and solvents. Incorrect, 2 attempts remaining H & H H Select to Add Arrows H₂O heat H :O: :Br: H H Select to Add Arrows H₂O heat Select to Draw Productarrow_forwardtu Please don't provide handwritten solutionarrow_forward
- Curved arrows are used to illustrate the flow of electrons. Using the provided starting structure, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. Then draw any missing organic intermediates or products for this reaction. Include all lone pairs in the structures. Ignore inorganic byproducts and counterions. H 0:0- HsC H Select to Add Arrows H HaC Select to Add Arrows CH₂OH, H CH3OH, H* CH3OH, H* HaC H Select to Add Arrows 1 CH3OH, H+ HaC H H Select to Add Arrows Iarrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting structure, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. Then draw the organic product of this reaction. Include all lone pairs in the structures. Ignore inorganic byproducts, counterions, and solvents. No@ I Select to Add Arrows NaCN DMSO Atoms. Bonds and Rings Charges and Lone Pairs Drawing NH 6 NH Undo Reset Remove Done Drag To Paarrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting structure, draw the curved electron-pushing arrows for the following reaction or mechanistic steps. Be sure to account for all bond-breaking and bond-making steps. Then draw the organic product of this reaction. Include all lone pairs in the structures. Ignore inorganic byproducts and counterions. H. H H H H H H Select to Add Arrows CH3CH2ONa Select to Draw Product Na Ⓒarrow_forward
- Please give a detailed stepwise mechanism for the following reactions of Q.1 and Q.2. All arrows, charges, intermediates, and resonance structures must be shown.arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic steps. Be sure to account for all bond-breaking and bond-making steps. Na Ⓒ 0:0 H CH₂ Select to Add Arrows Na Ⓒ CH3arrow_forwardPredict Products. Using line structures, give the major product of each 1.[ reaction below. DON'T SHOW MECHANISMS HERE. Use scratch paper if needed. H2 a) Pt/C b) HBr H2SO4 HOarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY