
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
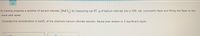
Transcribed Image Text:A chemist prepares a solution of barium chloride (BaCl,) by measuring out 87. g of barium chloride into a 350. mL volumetric flask and filling the flask to the
mark with water.
Calculate the concentration in mol/L of the chemist's barium chloride solution. Round your answer to 2 significant digits.
I mol
Expert Solution
arrow_forward
Step 1
Given:
Mass of BaCl2= 87 g
The volume of the solution, V=350 mL= 350/1000=0.350 L
[1 L = 1000 mL]
Molar mass of BaCl2=208.23 g/mol
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A chemist prepares a solution of barium chloride (BaCl,) by measuring out 48.6 g of barium chloride into a 200. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in mol/L of the chemist's barium chloride solution. Be sure your answer has the correct number of significant digits. I mol/Larrow_forwardA chemist prepares a solution of potassium dichromate (K,Cr,O,) by measuring out 35. g of potassium dichromate into a 300. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in mol/L of the chemist's potassium dichromate solution. Round your answer to 2 significant digits. alo | mol/Larrow_forwardA chemist prepares a solution of barium acetate BaC2H3O22 by measuring out 83.g of barium acetate into a 250.mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in /molL of the chemist's barium acetate solution. Round your answer to 2 significant digits.arrow_forward
- A chemist prepares a solution of sodium thiosulfate Na2S2O3 by measuring out 147.g of sodium thiosulfate into a 250.mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in /molL of the chemist's sodium thiosulfate solution. Round your answer to 3 significant digits.arrow_forwardA chemist prepares a solution of aluminum sulfate Al2SO43 by measuring out 100.g of aluminum sulfate into a 400.mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in /molL of the chemist's aluminum sulfate solution. Round your answer to 3 significant digits.arrow_forwardA chemist prepares a solution of sodium carbonate (Na₂CO3) by measuring out 13. g of sodium carbonate into a 200. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in mol/L of the chemist's sodium carbonate solution. Round your answer to 2 significant digits. mol/L Sarrow_forward
- A chemist prepares a solution of silver perchlorate (AgClO,) by weighing out 2.40 kg of silver perchlorate into a 500. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in g/dL of the chemist's silver perchlorate solution. Be sure your answer has the correct number of significant digits. x10 đLarrow_forwardA chemist prepares a solution of silver nitrate (AGNO,) by weighing out 155. g of silver nitrate into a 150. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in g/dL of the chemist's silver nitrate solution. Be sure your answer has the correct number of significant digits. x10arrow_forwardO MEASUREMENT Adding or subtracting and multiplying or dividing measurements A chemistry student must write down in her lab notebook the concentration of a solution of sodium hydroxide. The concentration of a solution equals the mass of what's dissolved divided by the total volume of the solution. Here's how the student prepared the solution: • The label on the graduated cylinder says: empty weight: 1.5 g • She put some solid sodium hydroxide into the graduated cylinder and weighed it. With the sodium hydroxide added, the cylinder weighed 36.669 g. • She added water to the graduated cylinder and dissolved the sodium hydroxide completely. Then she read the total volume of the solution from the markings on the graduated cylinder. The total volume of the solution was 37.11 mL. What concentration should the student write down in her lab notebook? Be sure your answer has the correct number of significant digits. g mL Explanation -1 Check 99+ 0/5 VUDU © 2023 McGraw Hill LLC. All Rights…arrow_forward
- A chemist must prepare 0.675 L of 1.00 M aqueous nickel(II) chloride (NiCl,) working solution. She'll do this by pouring out some 4.31 mol aqueous L nickel(II) chloride stock solution into a graduated cylinder and diluting it with distilled water. Calculate the volume in L of the nickel(II) chloride stock solution that the chemist should pour out. Round your answer to 3 significant digits. MAR W 3 MacBook Air DI DD 吕0 888 F9 F10 F7 F8 吕arrow_forwardA chemist prepares a solution of copper(II) fluoride (CuF;) by measuring out 1.31 mg of copper(11) fluoride into a 50 mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in mol/1. of the chemist's copper(II) fluoride solution. Be sure your answer has the correct number of significant digits. moll. OParrow_forwardA chemist prepares a solution of sodium chloride NaCl by measuring out 6.51mol of sodium chloride into a 150.mL volumetric flask and filling the flask to the mark with water. Calculate the concentration of the chemist's sodium chloride solution. Round your answer to 3 significant digits.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY