
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
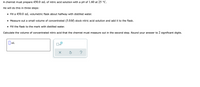
Transcribed Image Text:A chemist must prepare 450.0 mL of nitric acid solution with a pH of 1.60 at 25 °C.
He will do this in three steps:
• Fill a 450.0 mL volumetric flask about halfway with distilled water.
• Measure out a small volume of concentrated (5.0M) stock nitric acid solution and add it to the flask.
• Fill the flask to the mark with distilled water.
Calculate the volume of concentrated nitric acid that the chemist must measure out in the second step. Round your answer to 2 significant digits.
||mL
x10
?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A chemist must prepare 700.0 mL of sodium hydroxide solution with a pH of 12.30 at 25 °C. She will do this in three steps: • Fill a 700.0 mL volumetric flask about halfway with distilled water. • Weigh out a small amount of solid sodium hydroxide and add it to the flask. • Fill the flask to the mark with distilled water. Calculate the mass of sodium hydroxide that the chemist must weigh out in the second step. Round your answer to 2 significant digits. Ox10arrow_forwardCalculate the pH of the glacial acetic acid and the solution you prepared in step 1. Step 1: Glacial acetic acid, the most concentrated CH3COOH you can buy, has a concentration of 17.54 M. Calculate the concentration of CH3COOH is a solution that is prepared by combining 15.0 mL of glacial acetic acid with enough water to make 250-mL of solution. (Please be very detailed when answering the question)arrow_forwardWill 260.0 milligrams of calcium carbonate raise the pH of 75.0 milliliters of stomach fluid from a low pH of 1.00 into the normal pH range of 1.50-3.50? In the last discussion, you calculated that 260.0mg of calcium carbonate is 0.2600g of calcium carbonate. The next step is to figure out the formula of calcium carbonate and to calculate the molar mass of calcium carbonate in grams per mole. Submit a reply that that tells how you decided to approach the problem and shows the work that results in an answer. Use correct units and significant figures and correct symbols for elements and math. In addition, select another student's explanation that makes sense to you and reply with a comment about the insight you gained from their answer. Calcium carbonate is an ionic compound. Ionic nomenclature is in section 2.8. Molar mass is described on page 74 in section 2.8 of the textbook.arrow_forward
- 4arrow_forwardA chemist dissolves 576. mg of pure nitric acid in enough water to make up 150. mL of solution. Calculate the pH of the solution. Be sure your answer has the correct number of significant digits. 0 X Sarrow_forwardThe hydronium ion concentration in a sample of rainwater from a remote location is found to be 1.7 x 10−6 M at 25 °C. a) What is the concentration of hydroxide ions in the rainwater? b) What is the pH of the rainwater sample? c) Acid rain can have hydronium ion concentrations as high as 6.3 x 10-5 M. What is the pH of acid rain?arrow_forward
- A chemist dissolves 87. mg of pure barium hydroxide in enough water to make up 90. mL of solution. Calculate the pH of the solution. (The temperature of the solution is 25 °C.) Round your answer to 2 significant decimal places.arrow_forwardA chemist must prepare 600.0 mL of hydrobromic acid solution with a pH of 1.60 at 25 °C. He will do this in three steps: • Fill a 600.0 mL volumetric flask about halfway with distilled water. • Measure out a small volume of concentrated (6.0M) stock hydrobromic acid solution and add it to the flask. • Fill the flask to the mark with distilled water. Calculate the volume of concentrated hydrobromic acid that the chemist must measure out in the second step. Round your answer to 2 significant digits. 0₁ mL x10arrow_forwardPlease answer these questions on your Page 9 We dissolve 2.66 g of an unknown acid, HA, in enough water to produce 25.0 mL of solution. The pH of this solution is 1.11. We titrate this solution with a 0.166 M NaOH solution and we need 22.2 mL of this NaOH solution to reach the equivalence point. The temperature is 25.0°C throughout. (a) What is the molar mass of HA? (b) What is the dissociation constant, K₁, of A-(aq)? (c) What was the pH of the solution after the addition of the first 15.0 mL of the NaOH solution?arrow_forward
- A chemist must prepare 800.0 mL of potassium hydroxide solution with a pH of 12.80 at 25 °C. She will do this in three steps: • Fill a 800.0 mL volumetric flask about halfway with distilled water. • Weigh out a small amount of solid potassium hydroxide and add it to the flask. Fill the flask to the mark with distilled water. Calculate the mass of potassium hydroxide that the chemist must weigh out in the second step. Round your answer to 2 significant digits. ☐ g ☐ x10arrow_forwardQ M A chemist must prepare 900.0 mL of potassium hydroxide solution with a pH of 13.50 at 25 °C. She will do this in three steps: • Fill a 900.0 mL volumetric flask about halfway with distilled water. • Weigh out a small amount of solid potassium hydroxide and add it to the flask. . Fill the flask to the mark with distilled water. Calculate the mass of potassium hydroxide that the chemist must weigh out in the second step. Round your answer to 2 significant digits. 21,272 O ACIDS AND BASES Preparing a strong base solution with a given pH 2 8 W planation #3 Check E $ R X 3 n e % 5 T tvarrow_forwardPlease answer these questions on your Page 9 We dissolve 1.66 g of an unknown acid, HA, in enough water to produce 25.0 mL of solution. The pH of this solution is 1.33. We titrate this solution with a 0.277 M solution of NaOH and we need 14.4 mL of this NaOH solution to reach the equivalence point. The temperature is 25.0°C throughout. (a) ) What is the molar mass of HA? (b) ) What is the dissociation constant, Ka, of HA(aq)? (c) ( What is the pH at the equivalence point?arrow_forwardarrow_back_iosarrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY