
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question

Transcribed Image Text:A chemist measures the enthalpy change AH during the following reaction:
8 SO2(9) + 16 H,S(g)→ 3 Sg(s) + 16 H,0(1)
ДН--1863. kJ
Use this information to complete the table below. Round each of your answers to the nearest kJ/mol.
reaction
ΔΗ
x10
3
280, (2) + 4H,8 (g) → s,6) + 4H,0(1)
O kJ
3S,(s) + 16H,0(1)
–→ 880,(g) + 16H,S(g)
| kJ
6S, (s) + 32H,0(1)
1680, (g) + 32H,S(g)
O kJ
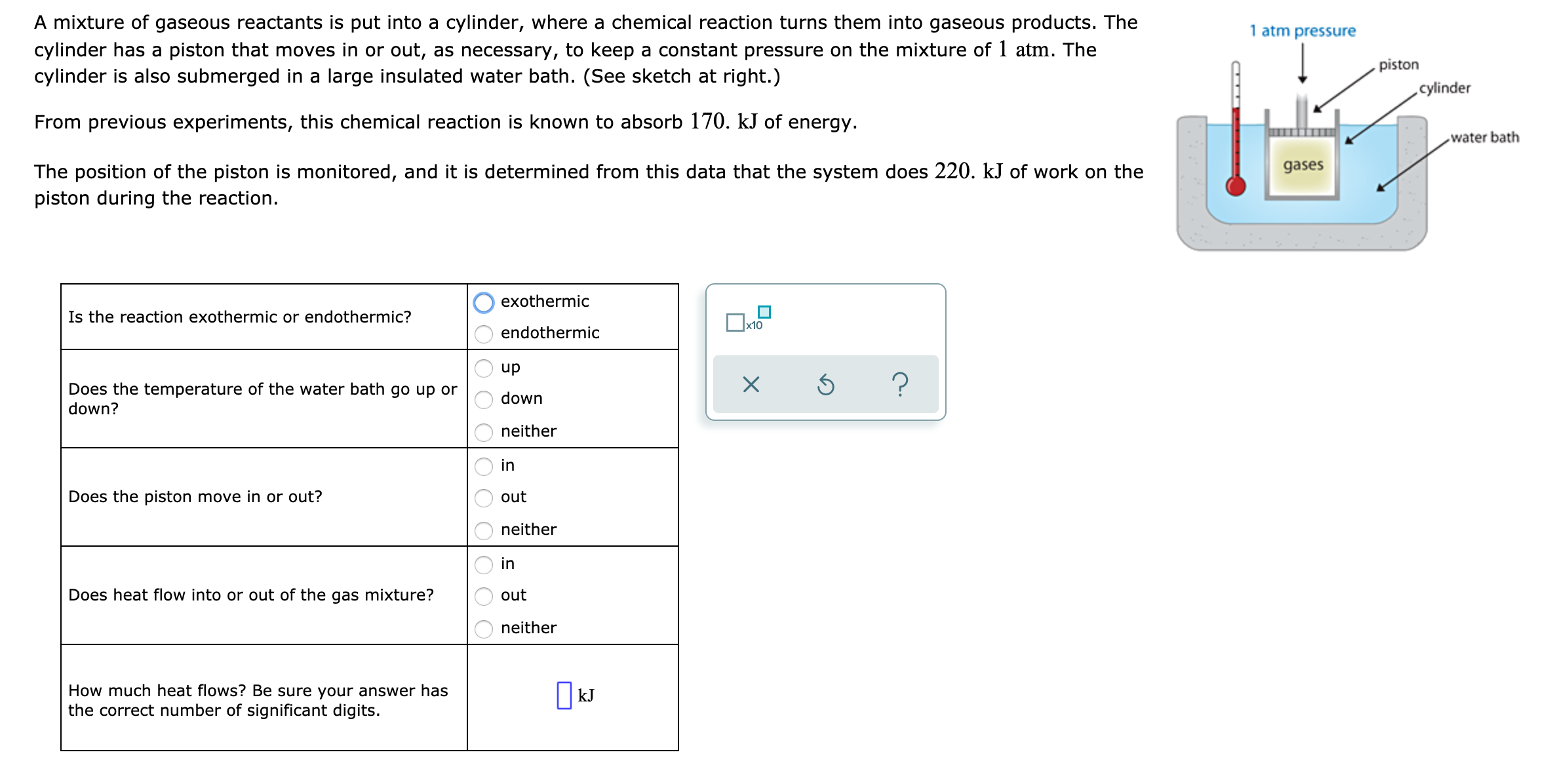
Transcribed Image Text:A mixture of gaseous reactants is put into a cylinder, where a chemical reaction turns them into gaseous products. The
1 atm pressure
cylinder has a piston that moves in or out, as necessary, to keep a constant pressure on the mixture of 1 atm. The
cylinder is also submerged in a large insulated water bath. (See sketch at right.)
piston
cylinder
From previous experiments, this chemical reaction is known to absorb 170. kJ of energy.
water bath
gases
The position of the piston is monitored, and it is determined from this data that the system does 220. kJ of work on the
piston during the reaction.
exothermic
Is the reaction exothermic or endothermic?
x10
endothermic
Does the temperature of the water bath go up or
down?
dn
down
neither
in
Does the piston move in or out?
out
neither
in
Does heat flow into or out of the gas mixture?
out
neither
How much heat flows? Be sure your answer has
the correct number of significant digits.
| kJ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Interpreting a heating curve.arrow_forwardAn industrial process for manufacturing sulfuric acid, H2SO4, uses hydrogen sulfide, H2S, from the purification of natural gas. In the first step of this process, the hydrogen sulfide is burned to obtain sulfur dioxide, SO2. 2H2S(g) + 3 O2(g) → 2 H2O(l) + 2 SO2(g); ∆H = -1124 kJ. The density of sulfur dioxide at 25 °C and 1.00 atm is 2.62 g/L, and the molar heat capacity is 30.2 J/mol °C. a) How much heat would be evolved in producing 1.00 L of SO2 at 25 °C and 1.00 atm? b) Suppose heat from this reaction is used to heat 1.00 L of SO2 from 25 °C to 500 °C for its use in the next step of the process. What percentage of the heat evolved is required for this?arrow_forwardthermometer A 53.2 g sample of quartz, which has a specific heat capacity of 0.730 J'g -1.°c-1 , is put into a calorimeter (see sketch at right) insulated container that contains 100.0 g of water. The temperature of the water starts off at 17.0 °C. When the temperature of the water stops changing it's 23.4 °C. The pressure remains constant at 1 atm. water Calculate the initial temperature of the quartz sample. Be sure your answer is rounded to the correct number of significant digits. sample a calorimeterarrow_forward
- The balanced combustion reaction for C,H, is 2 C,H,(1) + 15 O,(g) 12 CO, (g) + 6 H, O(1) + 6542 kJ If 8.700 g C,H, is burned and the heat produced from the burning is added to 5691 g of water at 21 °C, what is the final 9. temperature of the water?arrow_forwardThe heater used in a 4.32 m×3.69 m×3.01 m4.32 m×3.69 m×3.01 m dorm room uses the combustion of natural gas (primarily methane gas) to produce the heat required to increase the temperature of the air in the dorm room. Assuming that all of the energy produced in the reaction goes towards heating only the air in the dorm room, calculate the mass of methane required to increase the temperature of the air by 5.30 °C5.30 °C. Assume that the specific heat of air is 30.0 J/K·mol30.0 J/K·mol and that 1.00 mol1.00 mol of air occupies 22.4 L22.4 L at all temperatures. Enthalpy of formation values can be found in this table. Assume gaseous water is produced in the combustion of methane.arrow_forwardA mixture of gaseous reactants is put into a cylinder, where a chemical reaction turns them into gaseous products. The cylinder has a piston that moves in or out, as necessary, to keep a constant pressure on the mixture of 1 atm. The cylinder is also submerged in a large insulated water bath. (See sketch at right.) 1 atm pressure pist From previous experiments, this chemical reaction is known to absorb 278. kJ of energy. The temperature of the water bath is monitored, and it is determined from this data that 285. kJ of heat flows into the system during the reaction. gases O exothermic Is the reaction exothermic or endothermic? O endothermic O up Does the temperature of the water bath go up or down? O down O neither in Does the piston move in or out? O out O neither O does work Does the gas mixture do work, or is work done on it? O work is done on it O neither How much work is done on (or by) the gas mixture? Round your answer to 1 significant digit.arrow_forward
- A 140-g aluminum cylinder is removed from a liquid nitrogen bath, where it has been cooled to - 196∘C . The cylinder is immediately placed in an insulated cup containing 90.0 g of water at 15.0 ∘C . If your answer is 0 ∘C , determine the amount of water that has frozen.arrow_forwardA coin dealer, offered a rare silver coin, suspected that it might be a counterfeit nickel copy. The dealer heated the coin, which weighed 14.5 g to 100°C in boiling water and then dropped the hot coin into 25.0 g of water at T = 15.0°C in an insulated coffee-cup, and measured the rise in temperature. If the coin was really made of silver, what would the final temperature of the water be (in °C)? (for nickel, s = 0.445 J/g°C; for silver, s = 0.233 J/g°C )arrow_forward3 A hot air balloon will lie flat until a heat source is initiated, Once enough heat is produced the balloon begins to inflate. What process is responsible for a hot air bailloon's inflation? Thermal energy will cause hydrogen in A the air to change into helium, which causes the balloon to inflate, Thermal energy from the heat source will B heat the fabric of the balloon, which causes the balloon to inflate. Thermal energy will move from the surrounding air to the heat source which causes air to sink into the balloon, which causes the balloon to inflate. Thermal energy will move from the heat source to surrounding cooler air which causes the air to rise until uniform temperature has been reached, which causes the balloon to inflate.arrow_forward
- Please help me answer this thermochemistry question (i keep getting it wrong and this is my last try )arrow_forwardA 10.7 g sample of aluminum at 85.3 0C is added to 117 mL of water initially at 14.6 0C. Assuming no heat is lost to the surroundings, calculate the final temperature of the system.arrow_forwardChaeyoung wants to study the heat transfer between a 50 g of heated block of iron (cFe = 0.449 J/g ⁰C) at 500 ⁰C and 100 g of water (cH2O = 4.184 J/g ⁰C) at 10 ⁰C using a calorimeter. Find qH2O and qFe.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY