
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
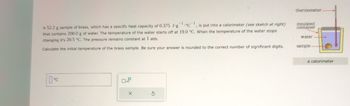
Transcribed Image Text:thermometer-
A 52.2 g sample of brass, which has a specific heat capacity of 0.375 J-g1C1, is put into a calorimeter (see sketch at right)
that contains 200.0 g of water. The temperature of the water starts off at 19.0 °C. When the temperature of the water stops
changing it's 20.5 °C. The pressure remains constant at 1 atm.
Calculate the initial temperature of the brass sample. Be sure your answer is rounded to the correct number of significant digits.
insulated
container
water
sample
☐ °C
X
5
a calorimeter
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- A sample of brass, which has a specific heat capacity of 0.375 J.g •°C is put into a calorimeter (see sketch at right) that contains 250.0 g of water. The brass sample starts off at 86.8 °C and the temperature of the water starts off at 24.0 °C. When the temperature of the water stops changing it's 25.1 °C. The pressure remains constant at 1 atm. Calculate the mass of the brass sample. Be sure your answer is rounded to 2 significant digits. g x10 thermometer. insulated container water sample a calorimeterarrow_forward100.0 mL of cold water at 20.5 oC was poured into a styrofoam coffee cup calorimeter. After adding 100.0 mL of hot water at 30.5 oC and mixing it thoroughly, the temperature of the mixture was found to 24.7 oC. Find the heat capacity of the calorimeter. [Specific heat capacity of water is 4.184 J/goC. Assume density of water is 1.0 g/mL at both temperatures.]arrow_forwardthermometer insulated container water A 57.9 g sample of quartz, which has a specific heat capacity of 0.730 J-g¹c¹, is put into a calorimeter (see sketch at right) that contains 300.0 g of water. The temperature of the water starts off at 21.0 °C. When the temperature of the water stops changing it's 23.3 °C. The pressure remains constant at 1 atm. Calculate the initial temperature of the quartz sample. Be sure your answer is rounded to the correct number of significant digits. sample 0°C a calorimeterarrow_forward
- A 57.0g sample of aluminum is put into a calorimeter (see sketch at right) that contains 250.0g of water. The aluminum sample starts off at 96.9°C and the temperature of the water starts off at 23.0°C. When the temperature of the water stops changing it's 26.2°C. The pressure remains constant at 1atm. Calculate the specific heat capacity of aluminum according to this experiment. Be sure your answer is rounded to 2 significant digits. Please type answer note write by hend.arrow_forwardA 56.2 g sample of aluminum, which has a specific heat capacity of 0.897 J-g¹C¹, is put into a calorimeter (see sketch at right) that contains 250.0 g of water. The temperature of the water starts off at 24.0 °C. When the temperature of the water stops changing it's 26.9 °C. The pressure remains constant at 1 atm. Calculate the initial temperature of the aluminum sample. Be sure your answer is rounded to the correct number of significant digits. °C X Ś thermometer- insulated. container water sample a calorimeterarrow_forwardthermometer- A sample of polystyrene, which has a specific heat capacity of 1.880 J-g¹C¹, is put into a calorimeter (see sketch at right) that insulated container contains 200.0 g of water. The polystyrene sample starts off at 85.8 °C and the temperature of the water starts off at 16.0 °C. When the temperature of the water stops changing it's 23.1 °C. The pressure remains constant at 1 atm. water. Calculate the mass of the polystyrene sample. Be sure your answer is rounded to 2 significant digits. g x10 X 5 ? sample a calorimeter doarrow_forward
- thermometer A 58.2 g sample of polystyrene is put into a calorimeter (see sketch at right) that contains 300.0 g of water. The polystyrene insulated container sample starts off at 89.1 °C and the temperature of the water starts off at 19.0 °C. When the temperature of the water stops changing it's 23.0 °C. The pressure remains constant at 1 atm. water Calculate the specific heat capacity of polystyrene according to this experiment. Be sure your answer is rounded to the correct number of significant digits. sample a calorimeter J Ox10 g.°C ?arrow_forwardA sample of iron, which has a specific heat capacity of 0.449 J-g¹C¹, is put into a calorimeter (see sketch at right) that contains 150.0 g of water. The iron sample starts off at 85.1 °C and the temperature of the water starts off at 22.0 °C. When the temperature of the water stops changing it's 24.3 °C. The pressure remains constant at 1 atm. Calculate the mass of the iron sample. Be sure your answer is rounded to 2 significant digits. 08 X S thermometer. insulated container water- sample a calorimeter 區 dearrow_forwardthermometer A 53.6 g sample of brass, which has a specific heat capacity of 0.375 J'g.°C', is put into a calorimeter (see sketch at right) that contains 300.0 g of water. The temperature of the water starts off at 19.0 °C. When the temperature of the water stops changing it's 20.2 °C. The pressure remains constant at 1 atm. insulated container water Calculate the initial temperature of the brass sample. Be sure your answer is rounded to 2 significant digits. sample - a calorimeter Continue Submit Assignment 02021 McGraw Hill LLC A Rights Reserved. Terms of Use Privacy Center I Accessibilityarrow_forward
- A hot 101.7101.7 g lump of an unknown substance initially at 184.9184.9 °C is placed in 35.0 mL of water initially at 25.0 °C and the system is allowed to reach thermal equilibrium. The final temperature of the system is 86.486.4 °C. Substance Specific heat (J/(g·°C)) aluminum 0.897 graphite 0.709 rhodium 0.243 titanium 0.523 tungsten 0.132 zinc 0.388 water 4.184 Using this information and the specific heat values for several metals in the table, identify the unknown substance. Assume no heat is lost to the surroundings. a) zinc b) graphite c) titanium d) tungsten e) rhodium f) aluminumarrow_forward- 1 A sample of glass, which has a specific heat capacity of 0.670 J-g.°C¯', is put into a calorimeter (see sketch at right) that contains 150.0 g of water. The glass sample starts off at 94.1 °C and the temperature of the water starts off at 24.0 °C. When insulated container the temperature of the water stops changing it's 28.2 °C. The pressure remains constant at 1 atm. water dlo Calculate the mass of the glass sample. Be sure your answer is rounded to the correct number of significant digits. sample 18 Ar a calorimeter x10arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY