
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
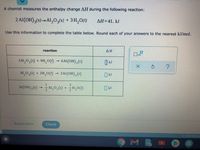
Transcribed Image Text:A chemist measures the enthalpy change AH during the following reaction:
2 Al(OH),(s)-Al,0,(s) + 3H,0(1)
AH=41. kJ
Use this information to complete the table below. Round each of your answers to the nearest kJ/mol.
reaction
ΔΗ
3AI,0,(s) + 9H,0()
6Al(OH),(s)
Al,0,(s) + 3H,0()
2Al (OH), (s)
Al(OH),(6) - Al,0,(0) + ,00
1
Al,0,(5) + H,0()
O kJ
Explanation
Check
2021 McGra
M B
Expert Solution
arrow_forward
Step 1
Enthalpy change (∆H) of a reaction changes as the thermochemical equation changes in the following ways
1. If the chemical equation is reversed then sign of ∆H is reversed .
2. If chemical equation is multiplied with a number then ∆H is also multiplied with same number
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Here is another question that is similar to the last that I do not know where to start: When a 15.44 g sample of solid NH4NO3 dissolves in 150.00 g of water in a coffee-cup calorimeter, the temperature decreases from 291.75 K to 289.35 K. Calculate the value of ΔH (in kJ/mol) for the reaction and determine if the reaction is endothermic or exothermic. **Assume the specific heat capacity of the solution is that of pure water (Cs = 4.184 J/g∙K).arrow_forwardA chemist carefully measures the amount of heat needed to raise the temperature of a 1.51 kg sample of C,H,N from 44.7 °C to 57.9 °C. The experiment shows that 4.02 × 10* J of heat are needed. What can the chemist report for the molar heat capacity of C,H,N? Round your answer to 3 significant digits. OJ-mol 1 - 1 ·K х10arrow_forwardThe enthalpy of solution (dissolving) of sodium hydroxide is given below. Determine the change in temperature of a coffee cup calorimeter containing 150 ml of water when 5.00 g NaOH (40.00 g/mol) is added to the container. You may assume that the solution has the same specific heat as water. NaOH(s) --> NaOH(aq) ∆H = -44.51 KJarrow_forward
- Using the equations 2 H: (g) + O2 (g) → 2 H2O (I) AH° = -572 kJ/mol Ca (s) + ½ O2 (g) → CaO (s) AH° = -635 kJ/mol CaO (s) + H:O (1) → Ca(OH): (s) AH° = -64 kJ/mol Determine the enthalpy (in kJ/mol) for the reaction Ca (s) + 2 H2O (1) → Ca(OH)2 (s) + H2 (g).arrow_forwardIn a coffee-cup calorimeter, 110.0 mL of 1.3 M and 110.0 mL of 1.3 M are mixed. Both solutions were originally at 22.2°C. After the reaction, the final temperature is 30.9°C. Assuming that all the solutions have a density of 1.0 and a specific heat capacity of 4.18 J/°C·g, calculate the enthalpy change for the neutralization of HCl by NaOH. Assume that no heat is lost to the surroundings or to the calorimeter.arrow_forwardi need help with this questionarrow_forward
- 3. 50.0 mL of 1.0 M sodium hydroxide and 50.0 mL of 1.0 M hydrochloric acid, both at 22.5 °C, were mixed together in a calorimeter. The temperature increased to 29.2 °C. Determine enthalpy of the reaction. You may assume that both solutions have the same density and heat capacity of water (d = 1.00 g/mL, SH=4.184 J/g°C). Remember, AH = q at constant pressure.arrow_forwardIn a coffee-cup calorimeter, 110.0 mL of 1.4 M NaOH and 110.0 mL of 1.4 M HCl are mixed. Both solutions were originally at 24.0°C. After the reaction, 3 the final temperature is 33.4°C. Assuming that all the solutions have a density of 1.0 g/ cm° and a specific heat capacity of 4.18 J/°C.g, calculate the enthalpy change for the neutralization of HCl by NaOH. Assume that no heat is lost to the surroundings or to the calorimeter. ΔΗ - kJ/molarrow_forwardA chemist carefully measures the amount of heat needed to raise the temperature of a 0.19 kg sample of C,H,N from -4.5 °C to 6.6 °C. The experiment shows that 6.14 × 10° J of heat are needed. What can the chemist report for the molar heat capacity of C,H,N? Round your answer to 2 significant digits. O J-mol -1 - 1 ·K х10 ?arrow_forward
- Section 5.6 You place 0.0750 g of magnesium chips in a coffee-cup calorimeter and then add 100.0 mL of 1.00 M HCI. The reaction that occurs is: -> Mg (s) + 2 HCI (aq) → H2 (g) + MgCl2 (aq) The temperature of the solution raises from 20.15 °C to 23.29 °C. What is the enthalpy change or the reaction per mole of Mg? Assume that the specific heat capacity of the solution is 4.20 J/g K and the density of the solution is 1.00 g/mL. 412 kJ/mol 428 kJ/mol -412 kJ/mol -428 kJ/molarrow_forwardA chemist wastes a delicious brownie by burning it in a bomb calorimeter. Before burning, the brownie weighed 18.19 grams. During the combustion process in the bomb calorimeter, the heat of the calorimeter increased by 8.75 ∘C. The known heat capacity of the calorimeter that was used was 33.53 kJ/∘C. What is the energy content, in kJ/g, of this brownie? Enter your answer with no units.arrow_forwardCalcium hydroxide is prepared by adding calcium oxide to water: CaO (s) + H2O (l) → Ca(OH)2 (s) Calculate the enthalpy change (ΔH) for this reaction considering the following experiment. A 10.0 g sample of CaO (s) {MM = 56.08 g/mol} is added to 1.00 liter of water in a calorimeter with a total heat capacity of 4.37 kJ K-1 and the temperature increases by 2.70 K.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY