
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
A chemist is working on a new kind of antacid the chemist mixed two powdery substances together in a sealed container nothing escaped witch of these diagrams shows the repeating grouping of atoms that make up the ending substances
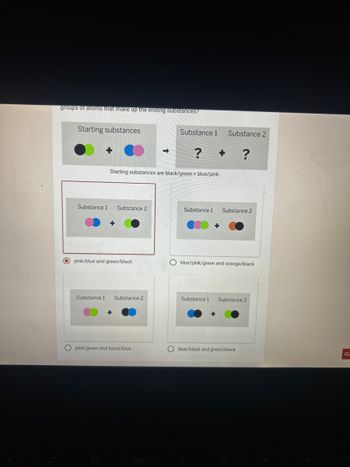
Transcribed Image Text:groups of atoms that make up the ending substances?
Starting substances
Substance 1 Substance 2
+
?
+
?
Starting substances are black/green + blue/pink.
Substance 1 Substance 2
Substance 1 Substance 2
+
+
pink/blue and green/black
blue/pink/green and orange/black
Substance 1 Substance 2
Substance 1 Substance 2
pink/green and black/blue
blue/black and green/black
CL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- In the ionic compound formed between silver and nitrate, how many of each type of ion is/are present? silver nitrate ※一 F11 F3 F4 F5 F6 F7 F8 F9 F10 & * 7 8. Y U G J K C V N く この エarrow_forwardThe ion question is an example. You are not answering that question. It's just an example of how your answer should look for the other question. Use evidence from the text and try to be detailed you can include prior knowledge you may know as well.arrow_forwardA large number of atoms of the same kind is called an element a compound a mixture Depends on the number of atoms None of thesearrow_forward
- Complete the table below by writing the symbols for the cation and anion that make up each ionic compound. The first row has been completed for you. ionic compound cation NaCl Co3(PO4)2 NH₂F FeBr₂ Fe(OH)3 Na 0 anion 0 X Sarrow_forwardImagine two new elements have been discovered with symbols Q and Z. Q is in group 2A and Z is in group 7a on the periodic table. What is the formula of the ionic compound that these two elements can form? Q₂Z₂ QZ₂ Q₂Z QZ ZQ₂ O Z₂Q2 O Z₂Qarrow_forwardWhat would you name the following: H3AwO3arrow_forward
- Complete the table below by writing the symbols for the cation and anion that make up each ionic compound?.arrow_forwardComplete the table below by writing the symbols for the cation and anion that make up each ionic compound. The first row has been completed for you. cation anion NaCl Na Cl × ionic compound Co. (PO) Cul₂ Fe2(SO₂), V Br 3 ☐ U ☐ ☐arrow_forwardionic compound cation anion NaCl Na Cl × 딤 Complete the table below by writing the symbols for the cation and anion that make up each ionic compound. The first row has been completed for you. Cr(OH), NH⭑F MnO, CoBr₂ Uarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY