
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
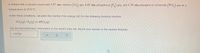
Transcribed Image Text:A chemist fills a reaction vessel with 5.97 atm chlorine (Cl,) gas, 6.65 atm phosphorus (P gas, and 1.79 atm phosphorus tríchloride (PCI,) gas at a
temperature of 25.0°C.
Under these conditions, calculate the reaction free energy AG for the following chemical reaction:
6Cl,(2) + P,(g) = 4PCI, (3)
Use the thermodynamic information in the ALEKS Data tab. Round your answer to the nearest kilojoule.
-1107|1J
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A closed vessel of volume 2.1 L contains a mixture of neon and fluorine gas. The total pressure of the mixture in the vessel is 3.8 atm at 0.0 °C. When the mixture is heated to 15.0 °C, the entropy of the mixture increases by 0.338 J · K-'. Calculate the number of moles of neon and fluorine in the mixture. mol NNe mol NF2 IIarrow_forwardA chemist fills a reaction vessel with 0.855 g silver chromate (Ag₂ CrO4) solid, 0.779 M silver (Ag*) aqueous solution, and 0.830 M chromate (Cro₂²-) aqueous solution at a temperature of 25.0°C. Under these conditions, calculate the reaction free energy AG for the following chemical reaction: 2- Ag₂ CrO4(s) ⇒ 2Ag+ (aq) + CrO² (aq) Use the thermodynamic information in the ALEKS Data tab. Round your answer to the nearest kilojoule. KJ Xarrow_forwardA chemist fills a reaction vessel with 3.33 atm chlorine (C1₂) gas, 9.77 atm phosphorus (P4) gas, and 6.02 atm phosphorus trichloride (PC13) gas at a temperature of 25.0°C. Under these conditions, calculate the reaction free energy AG for the following chemical reaction: 6C1₂(g) +P4 (g) = 4PC13 (g) Use the thermodynamic information in the ALEKS Data tab. Round your answer to the nearest kilojoule.arrow_forward
- 3.65 g of zinc metal react according tyo the following reaction at 25 C. Is this reaction spontaneous? Zn (0 + 2H^+ (aq) -> zn^2= (aq) + H2 (g)arrow_forwardPlease don't provide handwritten solution ...arrow_forwardA chemist fills a reaction vessel with 2.90 atm nitrogen monoxide (NO) gas, 6.95 atm chlorine (Cl,) gas, and 7.94 atm nitrosyl chloride (NOCI) gas at a temperature of 25.0°C. Under these conditions, calculate the reaction free energy AG for the following chemical reaction: 2NO (g) + Cl, (g) 2NOC1 (g) Use the thermodynamic information in the ALEKS Data tab. Round your answer to the nearest kilojoule.arrow_forward
- A chemist fills a reaction vessel with 1.75 atm nitrogen monoxide (NO) gas, 1.94 atm chlorine (Cl₂) gas, and 4.19 atm nitrosyl chloride (NOCI) gas at a temperature of 25.0°C. Under these conditions, calculate the reaction free energy AG for the following chemical reaction: 2NO(g)+Cl₂(g) 2NOCI(g) Use the thermodynamic information in the ALEKS Data tab. Round your answer to the nearest kilojoule.arrow_forwardThe standard reaction free energy AG° = 29. kJ for this reaction: Fe2O3(g) + 2H2 (g) --> 2 Fe(s) + 3H2O(l) Use this information to complete the table below. Round each of your answers to the nearest kJ.arrow_forwardA chemist fills a reaction vessel with 3.93 atm methane (CH,) gas, 1.61 atm oxygen (O,) gas, 5.89 atm carbon dioxide (CO,) gas, and 4.78 atm hydrogen (H2) gas at a temperature of 25.0°C. Under these conditions, calculate the reaction free energy AG for the following chemical reaction: CH, (g) +0,(g) CO,(g)+2H, (g) Use the thermodynamic information in the ALEKS Data tab. Round your answer to the nearest kilojoule. || kJarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY