
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN: 9781305580343
Author: Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
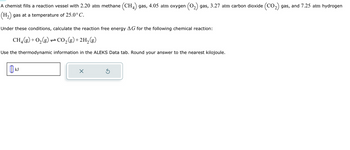
Transcribed Image Text:A chemist fills a reaction vessel with 2.20 atm methane (CH4) gas, 4.05 atm oxygen ₂) gas, 3.27 atm carbon dioxide (CO₂) gas, and 7.25 atm hydrogen
(H₂) gas at a temperature of 25.0°C.
Under these conditions, calculate the reaction free energy AG for the following chemical reaction:
CH₂(g) + O₂(g) → CO₂(g) + 2H₂(g)
Use the thermodynamic information in the ALEKS Data tab. Round your answer to the nearest kilojoule.
kJ
X
Ś
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 5 steps with 7 images

Knowledge Booster
Similar questions
- Adenosine triphosphate, ATP, is used as a free-energy source by biological cells. (See the essay on page 624.) ATP hydrolyzes in the presence of enzymes to give ADP: ATP(aq)+H2O(l)ADP(aq)+H2PO4(aq);G=30.5kJ/molat25C Consider a hypothetical biochemical reaction of molecule A to give molecule B: A(aq)B(aq);G=+15.0kJ/molat25C Calculate the ratio [B]/[A] at 25C at equilibrium. Now consider this reaction coupled to the reaction for the hydrolysis of ATP: A(aq)+ATP(aq)+H2O(l)B(aq)+ADP(aq)+H2PO4(aq) If a cell maintains a high ratio of ATP to ADP and H2PO4 by continuously making ATP, the conversion of A to B can be made highly spontaneous. A characteristic value of this ratio is [ATP][ADP][H2PO4]=500 Calculate the ratio [B][A] in this case and compare it with the uncoupled reaction. Compared with the uncoupled reaction, how much larger is this ratio when coupled to the hydrolysis of ATP?arrow_forwardIn muscle cells under the condition of vigorous exercise, glucose is converted to lactic acid (lactate),CH3CHOHCOOH, by the chemical reaction C6H12O6 2 CH3CHOHCOOHrG = 197 kJ/mol (a) If all of the Gibbs free energy from this reaction wereused to convert ADP to ATP, calculate how many molesof ATP could be produced per mole of glucose. (b) The actual reaction involves the production of 3 molATP per mole of glucose. Calculate the rG for thisoverall reaction. (c) Is the overall reaction in part (b) reactant-favored orproduct-favored?arrow_forwardExplain how the entropy of the universe increases when an aluminum metal can is made from aluminum ore. Thefirst step is to extract the ore, which is primarily a formof A12O3, from the ground. After it is purified by freeingit from oxides of silicon and iron, aluminum oxide ischanged to the metal by an input of electrical energy. 2Al2O3(s)electricalenergy4Al(s)+3O2(g)arrow_forward
- For the decomposition of formic acid, HCOOH(l)H2O(l)+CO(g) H = +29 kJ/mol at 25C. a Does the tendency of this reaction to proceed to a state of minimum energy favor the formation of water and carbon monoxide or formic acid? Explain. b Does the tendency of this reaction to proceed to a state of maximum entropy favor the formation of products or reactants? Explainarrow_forwardCobalt(II) chloride hexahydrate, CoCl26H2O, is a bright pink compound, but in the presence of very dry air it loses water vapor to the air to produce the light blue anhydrous salt CoCl2. Calculate the standard free-energy change for the reaction at 25C: CoCl26H2O(s)CoCl2(s)+6H2O(g) Here are some thermodynamic data at 25C: What is the partial pressure of water vapor in equilibrium with the anhydrous salt and the hexahydrate at 25C? (Give the value in mmHg.) What is the relative humidity of air that has this partial pressure of water? The relative humidity of a sample of air is Relativehumidity=partialpressureofH2O(g)inairvaporpressureofwater100 What do you expect to happen to the equilibrium partial pressure over the hexahydrate as the temperature is raised? Explain.arrow_forwardNatural gas, which is mostly methane, CH4, is a resource that the United States has in abundance. In principle, ethane can be obtained from methane by the reaction 2CH4(g)C2H6(g)+H2(g) (a) Calculate G° at 25°C for the reaction. Comment on the feasibility of this reaction at 25°C. (b) Couple the reaction above with the formation of steam from the elements: H2(g)+12O2(g)H2O(g)G=228.6kJ What is the equation for the overall reaction? Comment on the feasibility of the overall reaction.arrow_forward
- Calculate the standard Gibbs free-energy change when SO3 forms from SO2 and O2 at 298 K. Why is sulfur trioxide an important substance to study? (Hint: What happens when it combines with water?)arrow_forwardFor each of the following processes, identify the systemand the surroundings. Identify those processes that arespontaneous. For each spontaneous process, identify theconstraint that has been removed to enable the process to occur: Ammonium nitrate dissolves in water. Hydrogen and oxygen explode in a closed bomb. A rubber band is rapidly extended by a hangingweight. The gas in a chamber is slowly compressed by aweighted piston. A glass shatters on the floor.arrow_forwarda Calculate K1, at 25C for sulfurous acid: H2SO3(aq)H+(aq)+HSO3(aq) b Which thermodynamic factor is the most significant in accounting for the fact that sulfurous acid is a weak acid? Why?arrow_forward
- Elemental boron, in the form of thin fibers, can be made by reducing a boron halide with H2. BCl3(g) + 32 H2(g) B(s) + 3 HCl(g) Calculate rH, rS, and rG at 25 C for this reaction. Is the reaction predicted to be product-favored at equilibrium at 25 C? If so, is it enthalpy- or entropy-driven? [S for B(s) is 5.86 J/K mol.]arrow_forwardThe combustion of acetylene, C2H2, is a spontaneous reaction given by the equation 2C2H2(g)+5O2(g)4CO2(g)+2H2O(l) As expected for a combustion, the reaction is exothermic. What is the sign of H? What do you expect for the sign of S? Explain the spontaneity of the reaction in terms of the enthalpy and entropy changes.arrow_forwardThe free energy of formation of one mole of compound refers to a particular chemical equation. For each of the following, write that equation. a KBr(s) b CH3Cl(l) c H2S(g) d AsH3(g)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning