
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
What is the equilibrium constant at 25o C for the reaction
2 CO(g) + O2(g) ⇌ 2 CO2(g) given the following data:
ΔGfo[CO(g)] = –137.2 kJ/mol
ΔGfo[CO2(g)] = –394.4 kJ/mol
Expert Solution
arrow_forward
Given Information:
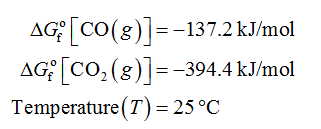
arrow_forward
Calculations:
The given reaction is shown below.
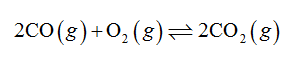
The Gibbs free change (ΔGorxn) for the given reaction is calculated as shown below.
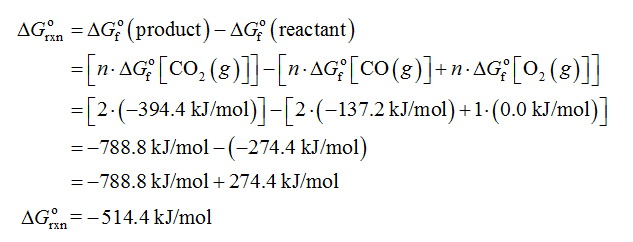
The conversion of temperature from oC to K is shown below.
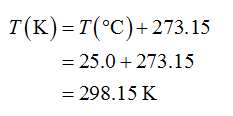
The equilibrium constant (Keq) for the above reaction is calculated by the formula shown below.
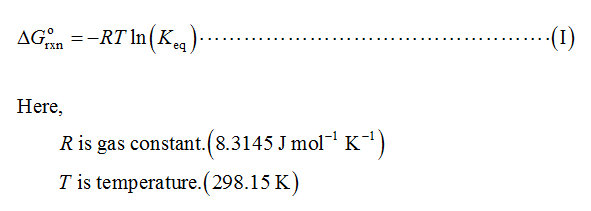
Substitute the given data in equation (I).
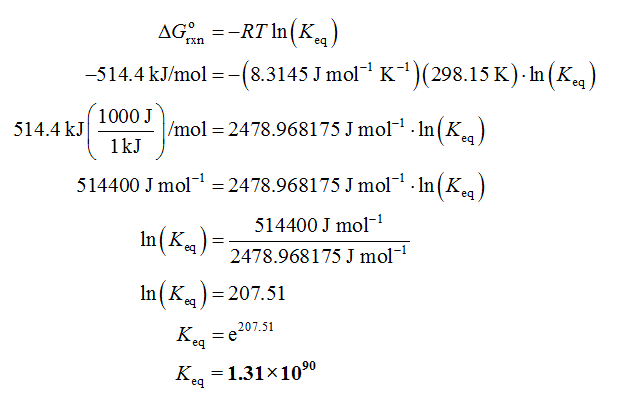
Therefore, the equilibrium constant (Keq) for the given reaction is 1.31 × 1090.
Step by stepSolved in 3 steps with 6 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- -) Phosphorous and chlorine gases combine to produce phosphorous trichloride: P2(g) + 3C12(g) → 2PC13(g) AG° at 298 K for this reaction is -642.9 kJ/mol. At 298 K for a reaction mixture that consists of 2.0 atm P2, 2.0 atm Cl and 1.0 atm PC13 what is the value of AG?arrow_forwardGiven equilibrium constant K = 0.051, please calculate the A,G° in unit of kJ for the following reaction at 489 °C, 2 HI(g) --> H2(g) + 12(g) (R = 8.314 J/K-mol). Please keep your answer to two decimal places.arrow_forwardConsider the following system at equilibrium where Ho = 18.8 kJ, and Kc = 9.52E-2, at 350 K: CH4(g) + CCl4(g) 2 CH2Cl2(g) If the VOLUME of the equilibrium system is suddenly decreased at constant temperature: whats the value of Kc The value of Q The reaction mustarrow_forward
- Consider the following equilibrium: N2 (g)+3H2(g)2NH3 (g) AG = -34. kJ Now suppose a reaction vessel is filled with 2.15 atm of nitrogen (N2) and 2.19 atm of ammonia (NH3) at 236. °C. Answer the following questions about this system: OO rise ☐ x10 fall OO Under these conditions, will the pressure of NH3 tend to rise or fall? Is it possible to reverse this tendency by adding H₂? In other words, if you said the pressure of NH3 will tend to rise, can that be changed to a tendency to fall by adding H2? Similarly, if you said the pressure of NH3 will tend to fall, can that be changed to a tendency to rise by adding H₂? If you said the tendency can be reversed in the second question, calculate the minimum pressure of H2 needed to reverse it. Round your answer to 2 significant digits. yes no ☐ atm Sarrow_forwardConsider the following equilibrium: 2NO, (g) – N,04(g) AG = - 5.4 kJ Now suppose a reaction vessel is filled with 7.26 atm of dinitrogen tetroxide (N,O) at 95. °C. Answer the following questions about this system: rise Under these conditions, will the pressure of N,O, tend to rise or fall? 4 fall Is it possible to reverse this tendency by adding NO,? In other words, if you said the pressure of N,0, will tend to rise, can that yes be changed to a tendency to fall by adding N0,? Similarly, if you said the no pressure of N,0, will tend to fall, can that be changed to a tendency to rise by adding NO,? If you said the tendency can be reversed in the second question, calculate the minimum pressure of NO, needed to reverse it. atm Round your answer to 2 significant digits. O Oarrow_forwardFor the following reaction, AH = 2816 kJ. 6 CO₂(g) + 6 H₂O(I) ⇒ C6H12O6(s) + 6 O₂(g) Select ONE combination of strategies that when applied to this system at equilibrium will result in an increase in the amount of C6H₁2O6 as equilibrium is re-established? a. Increasing Po₂ by adding more O2; increasing the temperature; decreasing the volume; or removing CO₂ b. Increasing Po₂ by adding more O₂ and decreasing the volume C. Decreasing the volume d. Increasing Po₂ by adding more O₂ e. Increasing the temperaturearrow_forward
- For the reaction below, what is the effect of raising the temperature? 4A(g) + B(g) 3C(g) AH = - 405 kJ Equilibrium shifts to the right; the reaction makes more products. O The reaction makes more of both products and reactants, so equilibrium is unaffected O Equilibrium re-establishes Equilibrium shifts to the left; the reaction makes more reactants.arrow_forwardWhat is AGxn (in kJ) at 1932 K for the following reaction? 2POCI3(g) → 2PC13(g) + O₂(g) POCI3(g): AH = -592.7 kJ/mol and Sº = 324.6 J/K mol) PC13(g): AH = -287.0 kJ/mol and So = 311.7 J/K mol) O₂(g): AH = ? kJ/mol and Sº = 205.0 J/K mol)arrow_forwardWe start with a sample of pure A(g). The following equilibrium is established 2 A(g) <---> B(g) + C(g) The total pressure is 8.33 atm and the temperature is 25.0oC. The partial pressure of A(g) is 5.26 atm. Calculate the value of the standard free enthalpy change (in kJ) of this reaction at 25.0oC.arrow_forward
- A reaction has an equilibrium constant (K) of 90.9. If the entropy of this reaction is -69.1 J/mol K at a temperature of 280 K, what is the enthalpy of this reaction in kJ/mol?Report your answer to one decimal place. ΔG = ΔH - T ΔSΔG = -RT ln Karrow_forwardConsider the decomposition of calcium carbonate: CaCO3 (s) ⇌ CaO(s) + CO2 (g) Calculate the pressure in atm of CO2 in an equilibrium process at 800oC. Assume that ΔHo = 177.8 kJ/mol and ΔSo = 160.5 J/molK under these conditions.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY