
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
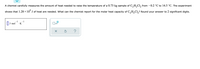
Transcribed Image Text:A chemist carefully measures the amount of heat needed to raise the temperature of a 0.73 kg sample of C,H,Cl, from -0.2 °C to 14.5 °C. The experiment
4
shows that 1.28 × 10" J of heat are needed. What can the chemist report for the molar heat capacity of C,H,Cl,? Round your answer to 2 significant digits.
- 1
- 1
J. mol
·K
x10
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A chemist carefully measures the amount of heat needed to raise the temperature of a 0.20 kg sample of C3H₂N from -7.2 °C to 6.9 °C. The experiment shows that 7.54 × 10³ J of heat are needed. What can the chemist report for the molar heat capacity of C₂H₁N? Be sure your answer has the correct number of significant digits. J. mol 1 .K - 1 x10 X Śarrow_forwardwhen 1 mole of iron metal reacts with hydrochloric acid at constant temperature and pressure to produce hydrogen gas and aqueous iron (II) chloride, 87.9 kJ or heat evolves. Write a thermochemical equation for this equation.arrow_forwardA 6.75g sample of gold (specific heat capacity of Au = 0.130 J/gC) is heated using 40.2 J of energy. If the original temperature of the gold sample is 25.0 degrees Celsius, what is its final temperature?arrow_forward
- An electrical heater is used to supply 205.0 J of energy to a 20.0 g sample of silver (specific heat is 0.237 J/(goC)), originally at 305.0 Kelvin. Determine the final temperature in Kelvin.arrow_forwardA 28.5 g piece of gold is heated and then allowed to cool. What is the change in temperature (°C) if the gold releases 0.103 kJ of heat as it cools? The molar heat capacity of gold is 25.4 J/mol・°C.arrow_forward0,11) G [References] Use the References to access important values if needed for this question. In the laboratory a student finds that it takes 58.2 Joules to increase the temperature of 11.7 grams of solid zinc from 25.0 to 38.6 degrees Celsius. The specific heat of zinc calculated from her data is J/g °C.arrow_forward
- The specific heat of a certain type of metal is 0.128 J/(g⋅∘C). What is the final temperature if 305 J of heat is added to 58.2 g of this metal, initially at 20.0 ∘C?arrow_forwardIn a calibration of a calorimeter, 50.3 g of water at 60.5 degrees C is added to 48.7g of water at 19.7 degrees C gives a mixture at 39.1 degrees C. What is the heat capacity of the calorimeter used in this experiment?arrow_forwardCalculate the energy required to heat 1.80kg of silver from 5.5°C to 19.1°C. Assume the specific heat capacity of silver under these conditions is 0.235J·g−1K−1. Round your answer to 3 significant digits.arrow_forward
- The specific heat of a certain type of metal is 0.128 J/(g.°C). What is the final temperature if 305 J of heat is added to 63.2 g of this metal, initially at 20.0 °C? °C Tfinal ||arrow_forwardThe temperature of an 6.95 g sample of an unknown metal increases from 22.0°C to 31.7°C upon the addition of 29.6 J of heat. The specific heat capacity of the metal is J/g-K.arrow_forward1 . A 28.5 g piece of gold is heated and then allowed to cool. What is the change in temperature (°C) if the gold releases 0.290 kJ of heat as it cools? The molar heat capacity of gold is 25.4 J/mol・°C. 2. 3.15 mol of an unknown solid is placed into enough water to make 150.0 mL of solution. The solution's temperature increases by 15.41°C. Calculate ∆H, in kJ/mol, for the dissolution of the unknown solid. (The specific heat of the solution is 4.184 J/g・°C and the density of the solution is 1.20 g/mL). 3. Using the equations 2 Fe (s) + 3 Cl₂ (g) → 2 FeCl₃ (s) ∆H° = -800.0 kJ/mol Si(s) + 2 Cl₂ (g) → SiCl₄ (s) ∆H° = -640.1 kJ/mol Determine the molar enthalpy (in kJ/mol) for the reaction 3 SiCl₄ (s) + 4 Fe (s) → 4 FeCl₃ (s) + 3 Si (s) 4. A sample of 2.30 g of potato chips was burned in a calorimeter. The calorimeter was calibrated to have a heat capacity of 6.94 kcal/°C. The heat released caused the temperature of the calorimeter to increase 1.75°C. Calculate the food caloric content of…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY