
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Please explain well

Transcribed Image Text:A charged particle enters the magnetic field of magnitude 0.35 T of a mass spectrometer at a speed of \(1.79 \times 10^6 \, \text{m/s}\). If it travels in a circle with a radius of 10.7 cm, find the mass-to-charge ratio and identify the particle from the table.
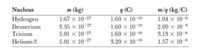
Transcribed Image Text:### Table: Characteristics of Atomic Nuclei
This table presents the mass, charge, and mass-to-charge ratio for various atomic nuclei, providing crucial information for understanding nuclear properties and reactions.
| Nucleus | Mass, \( m \) (kg) | Charge, \( q \) (C) | Mass/Charge, \( m/q \) (kg/C) |
|------------|---------------------|----------------------|-------------------------------|
| Hydrogen | \( 1.67 \times 10^{-27} \) | \( 1.60 \times 10^{-19} \) | \( 1.04 \times 10^{-8} \) |
| Deuterium | \( 3.35 \times 10^{-27} \) | \( 1.60 \times 10^{-19} \) | \( 2.09 \times 10^{-8} \) |
| Tritium | \( 5.01 \times 10^{-27} \) | \( 1.60 \times 10^{-19} \) | \( 3.13 \times 10^{-8} \) |
| Helium-3 | \( 5.01 \times 10^{-27} \) | \( 3.20 \times 10^{-19} \) | \( 1.57 \times 10^{-8} \) |
#### Explanation:
- **Nucleus**: Refers to the type of atomic nucleus considered.
- **Mass, \( m \)**: The mass of the nucleus expressed in kilograms.
- **Charge, \( q \)**: The electric charge of the nucleus expressed in coulombs.
- **Mass/Charge, \( m/q \)**: Represents the ratio of mass to charge, giving an indication of how mass is balanced by electric charge, which is significant for understanding behavior in magnetic and electric fields.
The table highlights differences in properties between isotopes and elements, critical for applications in nuclear physics and chemistry.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON