
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
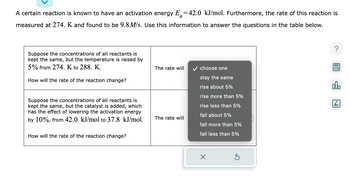
Transcribed Image Text:A certain reaction is known to have an activation energy E=42.0 kJ/mol. Furthermore, the rate of this reaction is
measured at 274. K and found to be 9.8M/s. Use this information to answer the questions in the table below.
Suppose the concentrations of all reactants is
kept the same, but the temperature is raised by
5% from 274. K to 288. K.
How will the rate of the reaction change?
Suppose the concentrations of all reactants is
kept the same, but the catalyst is added, which
has the effect of lowering the activation energy
by 10%, from 42.0 kJ/mol to 37.8 kJ/mol.
How will the rate of the reaction change?
The rate will
The rate will
✓ choose one
stay the same
rise about 5%
rise more than 5%
rise less than 5%
fall about 5%
fall more than 5%
fall less than 5%
?
00.
Ar
Expert Solution
arrow_forward
Step 1

the rate will rise by more than 5 % on increasing the temperature from 274 K to 288 K.
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Some measurements of the initial rate of a certain reaction are given in the table below. [H] 12| initial rate of reaction 1.04M 2.34M 17.0M/s 1.04M 1.10М 7.99 M/s 0.109 M 2.34М 1.78 M/s Use this information to write a rate law for this reaction, and calculate the value of the rate constant k. Round your value for the rate constant to 3 significant digits. Also be sure your answer has the correct unit symbol. rate = k I| x10 0 k =arrow_forward8. Smelling salt decomposes spontaneously at room temperature by the equation below. A student places a 10.00-g sample into a 1-liter sealed rigid vessel. What measurements should the student make to determine the rate of this reaction?(NH4)2CO3(s)→2NH3(g)+H2O(g)+CO2(g) a. change in mass and temperature b. temperature and time c. change in mass and time d. change in pressure and timearrow_forward4. A certain chemical reaction has a rate constant of 1.50 L/mol·min at 45.0°C with an activation energy of 108.0 kJ/mol. Calculate the value of the rate constant for the reaction at 60.0°C.arrow_forward
- Some measurements of the initial rate of a certain reaction are given in the table below. [N:] H2 initial rate of reaction 0.355M 2.28M 17.0M/s 0.355M 0.704M 1.62M/s 0.0958M 2.28M 4.59M/s Use this information to write a rate law for this reaction, and calculate the value of the rate constant k. Round your value for the rate constant to 3 significant digits. Also be sure your answer has the correct unit symbol. rate = k|| x10 k =arrow_forwardO KINETICS AND EQUILIBRIUM Deducing a rate law from initial reaction rate data Some measurements of the initial rate of a certain reaction are given in the table below. H, I, initial rate of reaction 0.735 M 0.260 M 78.0M/s 0.735 M 0.341 M 102. M/s 0.354 M 0.260 M 37.6M/s Use this information to write a rate law for this reaction, and calculate the value of the rate constant k. Round your value for the rate constant to 3 significant digits. Also be sure your answer has the correct unit symbol. Do rate = k| x10 k = Explanation Check © 2021 McGraw-Hill Educat IIarrow_forwardDeducing a rate law from initial reaction rate data Some measurements of the initial rate of a certain reaction are given in the table below. [N] [H] initial rate of reaction 0.335 M 2.47M 18.0M/s 0.335 M0.592M 1.03 M/s olo 0.521 M 2.47M 28.0 M/s Use this information to write a rate law for this reaction, and calculate the value of the rate constant k. Round your value for the rate constant to 3 significant digits. Also be sure your answer has the correct unit symbol. rate = k|| Ix10 k = ||arrow_forward
- The activation energy of the forward reaction 96.27 kJ/mol and the activation energy for the reverse reaction is 60.33 kJ/mol. What is the DH of the reaction? 78.3 kJ/mol 17.97 kJ/mol -156.6 kJ/mol 35.94 kJ/molarrow_forwardA certain catalyzed reaction is Known to have an activation energy Ea=29.0 kJ/mol. Furthermore, the rate of this reaction is measured at 331 K and found to be 2.7× 10* M/s. Use this information to answer the questions in the table below. Suppose the concentrations of all reactants is kept the same, but the temperature is raised by 10% from 331 K to 364 K. How will the rate or the reaction change? Suppose the concentrations of all reactants is kept the same, but the catalyst is removed which has the eftect of raising the activation energy by 10%, from 29.0 kJ/mol to 31.9 kJ mol How will the rate or the reaction change?arrow_forwardSome measurements of the initial rate of a certain reaction are given in the table below. [₂] [H₂] initial rate of reaction 2.38M 1.39 M 0.140 M/s 10.8M 1.39 M 2.88 M/s 2.38 M 0.584 M 0.0588 M/s Use this information to write a rate law for this reaction, and calculate the value of the rate constant k. Round your value for the rate constant to 3 significant digits. Also be sure your answer has the correct unit symbo rate = k ロ・ロ -0 Ś ? Continue k = Xarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY