
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question

Transcribed Image Text:A certain liquid X has a normal freezing point of -3.00 °C and a freezing point depression constant K, 4.23 °C-kg-mol. Calculate the freezing point of a
solution made of 72.3g of ammonium sulfate ((NH4), SO4) dissolved in 750. g of X.
Round you answer to 4 significant digits.
°C
X
3
∞o
E
olo
Ar
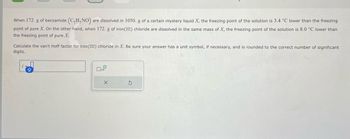
Transcribed Image Text:When 172. g of benzamide (C₂H,NO) are dissolved in 1050. g of a certain mystery liquid X, the freezing point of the solution is 3.4 °C lower than the freezing
point of pure X. On the other hand, when 172. g of iron (III) chloride are dissolved in the same mass of X, the freezing point of the solution is 8.0 °C lower than
the freezing point of pure X.
Calculate the van't Hoff factor for iron(III) chloride in X. Be sure your answer has a unit symbol, if necessary, and is rounded to the correct number of significant
digits.
X
S
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step 1: Q & A Guidelines and instructions
VIEW Step 2: Write the Formula of Depression in freezing point
VIEW Step 3: Calculate the number of moles of ammounium sulfate
VIEW Step 4: Determine molality of the solution
VIEW Step 5: Determine the depression in freezing point and then freezing point of the solution
VIEW Solution
VIEW Step by stepSolved in 6 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A certain liquid X has a normal boiling point of 105.30 °C and a boiling point elevation constant K₁ = 1.74 °C-kg-mol¯¹. Calculate the boiling point of a solution made of 8.59g of ammonium chloride (NHCl) dissolved in 150. g of X. Round you answer to 5 significant digits. пос x10 Xarrow_forwardThe boiling point of water is 100.0°C at 1 atmosphere. How many grams of silver acetate (166.9 g/mol), must be dissolved in 224.0 grams of water to raise the boiling point by 0.400 °C? mpor Refer to the table for the necessary boiling or freezing point constant. Solvent Kb (°C/m) Kf(°C/m) 0.512 1.22 Chloroform CHCl3 3.67 Benzene C6H6 2.53 Diethyl ether CH3 CH₂ OCH2 CH3 2.02 g silver acetate Water Ethanol Mass = this Formula H₂O CH3CH₂OH 1.86 1.99 5.12arrow_forwardA certain liquid X has a normal boiling point of 142.00 °C and a boiling point elevation constant K = 1.03 °C kg-mol A solution is prepared by dissolving some alanine (C₂H,NO₂) in 550. g of X. This solution boils at 143.2 °C. Calculate the mass of C3H-NO, that was dissolved. Round your answer to 2 significant digits. X Sarrow_forward
- Ethylene glycol (C₂H₆O₂) is used as an additive to the water in your automobile to lower its freezing point. A solution of ethylene glycol in water has a freezing point of -8.80°C. What is the mole fraction of ethylene glycol in this solution? (Kf for water is 1.86°C・kg/mol). Please use the correct significant figures. Thank you!arrow_forwardA solution of 4.63 g of anhydrous aluminum chloride (AlCl3) in 59.08 g of water freezes at -4.37 degrees celsius. Kf (AlCl3) =1.86 degrees celsius/m part 1: calculate the molar mass of AlCl3 from the formula. Round the answer to the hundredths place. ___ g/mol Part 2: Calculate the molar mass from the freezing point depression, assuming AlCl3 does not dissociate in ions. Round to 3 Significant digits. Part 3: Comparing the two values, how many ions will AlCl3 form? Round the answer to the nearest whole number.arrow_forwardA solution was prepared by dissovling 0.800 g of sulfur, S8, in 100.0 g of acetic acid, HC2H3O2. Calculate the freezing point and boiling point of the solution. Boiling point 118.5 degrees C. Freezing point 16.60 degrees C. Kb = 3.08 degrees C/m. Kf = 3.59 degrees C/m.arrow_forward
- Urea (NH2)2CO, is dissolved in 105.0 g of water. The solution freezes at -0.605°C. How many grams of urea were dissolved to make this solution? The Kf=1.858°C/m for water and its freezing point is 0.000°C. Express your answer to two decimal places.arrow_forwardNonearrow_forwardA certain liquid X has a normal freezing point of -9.00 °C and a freezing point depression constant K, = 7.04 °C-kg mol . A solution is prepared by dissolving some potassium bromide (KBr) in 400. g of X. This solution freezes at -13.6 °C. Calculate the mass of KBr that was dissolved. Round your answer to 2 significant digits.arrow_forward
- The freezing point of water is 0.00 °C at 1 atmosphere. How many grams of cobalt(II) acetate (177.0 g/mol), must be dissolved in 245.0 grams of water to reduce the freezing point by 0.300 °C? Refer to the table for the necessary boiling or freezing point constant. Solvent Formula Mass Water H₂O Ethanol CH3 CH₂OH 1.22 Chloroform CHC13 3.67 Benzene C6H6 2.53 Diethyl ether CH3 CH₂ OCH2 CH3 2.02 Kb (°C/m) Kf(°C/m) 0.512 g 1.86 1.99 5.12arrow_forwardWhat is the osmotic pressure in torr of a O.0173 M glucose solution at body temperature (37 °C)? What are the boiling and freezing points for the same solution? The osmotic pressure = i torr T= i °C Tbp= °C i [For this part, write your answer to the nearest ten-thousandth, rather than using significant figures rules.] P A O S MacBook Airarrow_forwardSolvent Formula Diethyl ether Water Ethanol Chloroform CHCl3 Benzene C6H6 H₂O CH3 CH₂ OH Kb (°C/m) K(°C/m) 0.512 The molality of the solution is The freezing point of the solution is 1.22 3.67 2.53 CH3 CH₂ OCH₂ CH3 2.02 m. 1.86 1.99 5.12 °C.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY