
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
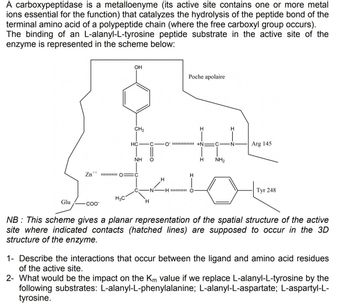
Transcribed Image Text:A carboxypeptidase is a metalloenyme (its active site contains one or more metal
ions essential for the function) that catalyzes the hydrolysis of the peptide bond of the
terminal amino acid of a polypeptide chain (where the free carboxyl group occurs).
The binding of an L-alanyl-L-tyrosine peptide substrate in the active site of the
enzyme is represented in the scheme below:
Glu
Zn++
COO™
OH
H3C
CH₂
HC
NH
IO C
H
H
Poche apolaire
-H
H
O+N: C N
H
H
H
NH₂
Arg 145
Туг 248
NB: This scheme gives a planar representation of the spatial structure of the active
site where indicated contacts (hatched lines) are supposed to occur in the 3D
structure of the enzyme.
1- Describe the interactions that occur between the ligand and amino acid residues
of the active site.
2- What would be the impact on the Km value if we replace L-alanyl-L-tyrosine by the
following substrates: L-alanyl-L-phenylalanine; L-alanyl-L-aspartate; L-aspartyl-L-
tyrosine.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Similar questions
- Many enzymes are switched "on" by attachment of a phosphate group at a specific serine somewhere on the protein (phosphorylation). The basic reaction is: E + ATP2 Ep + ADP Po SERINE PHOSPHO SERINC (Note the "squiggles" before the backone amide and carbonyl indicate the polypeptide chain continues on either side of the serine). For phosphorylation to have this effect, there has to be some equilibrium between inactive and active forms conformations of the enzyme: [Eactive] [Einactive] Einactive 2 Eactive; K* The same basic equilibrium must exist for the phosphorylated protein: [Ep,active] [Ep,inactive] EP,inactive 2 Ep,active; Kp = (a) If phosphorylation increases the measured activity of the enzyme, is K* or K larger? Why? (b) Does the phosphorylation site need to be near the site where the enzyme binds its substrate (e.g. the reactant whose chemistry it catalyzes)? Why or why not?arrow_forwardthe structures of chymotrypsin and other serine proteases revealed that the active sites of these enzymes shared a particular sterochemical arrangement of residues crucial to their activity. This came to be known as the catalytic triad, as it consisted of the oponyomous serine (Ser) residue, along with a histidine (His) and an aspartate (Asp) residue. Which of the following would result in the greatest decrease in function of the catalytic triad found in serine proteases? O Mutation to Ser, Cys, Asp O Mutation to Ser, His, Asp O Mutation to Cys, His, Asp O Mutation to Ser, His, Gluarrow_forwardWhich of the following statements are descriptions of metal ion catalysis or examples of metal ion catalysis? Choose all correct answers a Zn²+ cofactor may properly orient the substrate in the active site through ionic interactions. a covalent bond forms between enzyme and substrate lowers the energy or stabilizes the transition state or intermediate catalyst retains its original form after reaction occurs catalysts may participate in oxidation-reduction reactions by changes in the oxidation statearrow_forward
- The highest energy point of the serine protease reaction is the formation of the tetrahedral oxyanion intermediate on the original carbonyl carbon of the scissile peptide bond. True or False? DIPF is an irreversible inhibitor of serine proteases. True or False? The acyl-enzyme intermediate is the transition state of the reaction. True or False? Serine proteases are classified as isomerases. True or False?arrow_forwardWhat is the quaternary structure of glycogen phosphorylase? How would you characterize the allosteric effects of its small molecule substrate? (one word) What allosteric model (mechanism) that you have learned does it seem to follow? (one acronym)arrow_forwardWhat type of bonds in the tertiary structure of the enzyme break at high temperatures? Which ones will not break?arrow_forward
- The following molecules act as either inhibitors or activators of the enzyme that converts fructose-6-phosphate to fructose-1,6- diphosphate. Which do you think are activators? (A) ADP B AMP ATP citratearrow_forwardCompare and contrast the biological roles of the following amino acids the following pairs ofamino acids. Once you have documented these role state which member of the pair is most important and why.arrow_forwardThe enzyme which catalyzes the reaction below belongs to which enzyme classification? Н.С— ОН HC=0 C=0 Н-С—ОН H,C-0-P-o- || H,C –0-P-0- O Transfera Hydrolase Isomerase Oxidoreductasearrow_forward
- Some enzymes can be regulated by covalent modification, in which a group is covalently bonded to an amino acid side chain. Phosphorylation of side chains is a common regulatory covalent modification. In this essay, you will explore phosphorylation of side chains. Compare and contrast the types of interactions a free alcohol side chain such as serine could make with that of a phosphorylated alcohol such as phosphoserine (pSer). Could this modification affect the 3D structure of the protein? How? Imagine you are trying to separate a protein containing an unphosphorylated S residue from the same protein containing a pSer residue. Discuss how you could use ion-exchange chromatography to separate these two proteinsarrow_forwardCH₂OH 애 H2COPO 2- ATP OH 1 애 애 어 애 The class of the enzyme catalyzing the reaction shown in this figure is a(n) Choose the one best answer. ligase 매 +ADP 매arrow_forwardYou are trying to confirm that an enzyme you are studying has the following amino acid in its active site, and that this amino is critical for catalysis: HN- H3NⓇ Which of the following inhibitors could you use to provide evidence of the importance of this amino acid for enzyme function? Tetranitromethane 2,3-Butanedione Diethylpyrocarbonate lodoacetate Nevirapinearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON