Question
thumb_up100%
Needs Complete typed solution with 100 % accuracy.
Transcribed Image Text:You Answered
Correct Answer
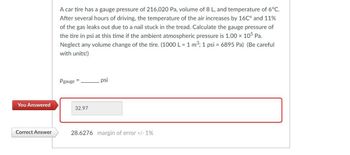
A car tire has a gauge pressure of 216,020 Pa, volume of 8 L, and temperature of 6°C.
After several hours of driving, the temperature of the air increases by 16℃° and 11%
of the gas leaks out due to a nail stuck in the tread. Calculate the gauge pressure of
the tire in psi at this time if the ambient atmospheric pressure is 1.00 x 105 Pa.
Neglect any volume change of the tire. (1000 L = 1 m³; 1 psi = 6895 Pa) (Be careful
with units!)
Pgauge
32.97
psi
28.6276 margin of error +/- 1%
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 13 images

Knowledge Booster
Similar questions
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios